The Anatomy of the Pelvic Floor and Sphincters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vulvodynia: a Common and Underrecognized Pain Disorder in Women and Female Adolescents Integrating Current
21/4/2017 www.medscape.org/viewarticle/877370_print www.medscape.org This article is a CME / CE certified activity. To earn credit for this activity visit: http://www.medscape.org/viewarticle/877370 Vulvodynia: A Common and UnderRecognized Pain Disorder in Women and Female Adolescents Integrating Current Knowledge Into Clinical Practice CME / CE Jacob Bornstein, MD; Andrew Goldstein, MD; Ruby Nguyen, PhD; Colleen Stockdale, MD; Pamela Morrison Wiles, DPT Posted: 4/18/2017 This activity was developed through a comprehensive review of the literature and best practices by vulvodynia experts to provide continuing education for healthcare providers. Introduction Slide 1. http://www.medscape.org/viewarticle/877370_print 1/69 21/4/2017 www.medscape.org/viewarticle/877370_print Slide 2. Historical Perspective Slide 3. http://www.medscape.org/viewarticle/877370_print 2/69 21/4/2017 www.medscape.org/viewarticle/877370_print Slide 4. What we now refer to as "vulvodynia" was first documented in medical texts in 1880, although some believe that the condition may have been described as far back as the 1st century (McElhiney 2006). Vulvodynia was described as "supersensitiveness of the vulva" and "a fruitful source of dyspareunia" before mention of the condition disappeared from medical texts for 5 decades. Slide 5. http://www.medscape.org/viewarticle/877370_print 3/69 21/4/2017 www.medscape.org/viewarticle/877370_print Slide 6. Slide 7. http://www.medscape.org/viewarticle/877370_print 4/69 21/4/2017 www.medscape.org/viewarticle/877370_print Slide 8. Slide 9. Magnitude of the Problem http://www.medscape.org/viewarticle/877370_print 5/69 21/4/2017 www.medscape.org/viewarticle/877370_print Slide 10. -

A Step Towards Stereotactic Navigation During Pelvic Surgery: 3D Nerve Topography
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository Surgical Endoscopy and Other Interventional Techniques https://doi.org/10.1007/s00464-018-6086-3 A step towards stereotactic navigation during pelvic surgery: 3D nerve topography A. R. Wijsmuller1,2 · C. Giraudeau3 · J. Leroy4 · G. J. Kleinrensink5 · E. Rociu6 · L. G. Romagnolo7 · A. G. F. Melani7,8,9 · V. Agnus2 · M. Diana3 · L. Soler3 · B. Dallemagne2 · J. Marescaux2 · D. Mutter2 Received: 10 May 2017 / Accepted: 1 February 2018 © The Author(s) 2018. This article is an open access publication Abstract Background Long-term morbidity after multimodal treatment for rectal cancer is suggested to be mainly made up by nerve- injury-related dysfunctions. Stereotactic navigation for rectal surgery was shown to be feasible and will be facilitated by highlighting structures at risk of iatrogenic damage. The aim of this study was to investigate the ability to make a 3D map of the pelvic nerves with magnetic resonance imaging (MRI). Methods A systematic review was performed to identify a main positional reference for each pelvic nerve and plexus. The nerves were manually delineated in 20 volunteers who were scanned with a 3-T MRI. The nerve identifiability rate and the likelihood of nerve identification correctness were determined. Results The analysis included 61 studies on pelvic nerve anatomy. A main positional reference was defined for each nerve. On MRI, the sacral nerves, the lumbosacral plexus, and the obturator nerve could be identified bilaterally in all volunteers. The sympathetic trunk could be identified in 19 of 20 volunteers bilaterally (95%). -

Uva-DARE (Digital Academic Repository)
UvA-DARE (Digital Academic Repository) Development of the pelvic floor : implications for clinical anatomy Wallner, C. Publication date 2008 Document Version Final published version Link to publication Citation for published version (APA): Wallner, C. (2008). Development of the pelvic floor : implications for clinical anatomy. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:27 Sep 2021 APPENDIX LETTERS TO THE EDITOR 183 184 BUTTOCK PAIN AFTER SACROSPINOUS HYSTEROPEXY Published as: Wallner C (2008). Buttock pain after sacrospinous hysteropexy. Int Urogynecol J Pelvic Floor Dysfunct. DOI: 10.1007/s00192-008-0646-3. 185 Dear Editor, With great interest I read the recent publication of Dietz et al [1] in your journal. The authors describe that buttock pain occurred post-operatively in ~18% of patients undergoing sacrospinous hysteropexy for pelvic organ prolapse. -

Pelvic Floor Physical Therapy for Vulvodynia a Clinician’S Guide
Pelvic Floor Physical Therapy for Vulvodynia A Clinician’s Guide Stephanie A. Prendergast, MPT KEYWORDS Vulvodynia Vestibulodynia Dyspareunia Pelvic floor dysfunction Pelvic floor physical therapy KEY POINTS Most women with complaints of vulvar pain have pelvic floor dysfunction. Pelvic floor screenings can be easily incorporated into a gynecology examination to iden- tify pelvic floor dysfunction. Successful treatment plans for vulvodynia are multimodal and include pelvic floor physical therapy. INTRODUCTION OF NEW NOMENCLATURE In 2003, the International Society for the Study of Vulvovaginal Disease (ISSVD) defined vulvodynia as ‘vulvar discomfort, most often described as burning pain, occurring in the absence of relevant visible findings or a specific, clinically identifiable, neurologic disorder’. This terminology served to acknowledge vulvar pain as a real disorder but fell short of classifying the syndrome as anything more than idiopathic pain. At that time, little was known about the pathophysiologic mechanisms that cause vulvodynia and treatment options were limited. Over the past decade, researchers have identified several causes of vulvodynia as well as associated factors/impair- ments. This identification resulted in the need to develop a new classification system to guide physicians toward better diagnosis and treatment. Last year the ISSVD, the International Society for the Study of Women’s Sexual Health, and the International Pelvic Pain Society came together to review the evidence and publish the 2015 Consensus Terminology -
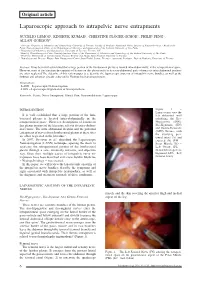
Laparoscopic Approach to Intrapelvic Nerve Entrapments
4-Lemos - Laparoscopic.qxp_treatment 05/03/18 12:11 Pagina 6 Original article Laparoscopic approach to intrapelvic nerve entrapments NUCELIO LEMOS 1, KINSHUK KUMAR 2, CHRISTINE PLÖGER-SCHOR 3, PHILIP PENG 4, ALLAN GORDON 5 1 Associate Professor of Obstetrics and Gynaecology University of Toronto, Faculty of Medicine Functional Pelvic Surgery & Neuropelveology - Head of the Pelvic Neurodysfunction Clinic of the Department of Obstetrics and Gynecology of the Federal University of São Paulo 2 Department of Obstetrics and Gynaecology, University of Toronto, Toronto, ON 3 Head of Physiotherapy in Pelvic Neurodysfunction Clinic of the Department of Obstetrics and Gynecology of the Federal University of São Paulo 4 Professor, Department of Anesthesiology Toronto Western and Mount Sinai Hospital University of Toronto 5 Neurologist and Director Wasser Pain Management Centre Sinai Health System, Toronto - Asscociate Professor, Dept of Medicine, University of Toronto Abstract: It has been well-established that a large portion of the lumbosacral plexus is located intra-abdominally, in the retroperitoneal space. However, most of the literature descriptions of lesions on this plexus refer to its extra-abdominal parts whereas its intra-abdominal portions are often neglected.The objective of this review paper is to describe the laparoscopic anatomy of intrapelvic nerve bundles, as well as the findings and advances already achieved by Neuropelveology practitioners. Abreviations: - LANN – Laparoscopic Neuronavigation - LION – Laparoscopic Implantation of Neuroprosthesis Keywords: Sciatic; Nerve Entrapment; Gluteal Pain; Neuromodulation; Laparoscopy. INTRODUCTION Figure 1. – Laparoscopic view the It is well established that a large portion of the lum - left abdominal wall bosacral plexus is located intra-abdominally in the exhibiting the Ilio- retroperitoneal space 1. -

The Innervation of the Pelvic Floor Muscles: a Reappraisal for the Levator Ani Nerve
UvA-DARE (Digital Academic Repository) Development of the pelvic floor : implications for clinical anatomy Wallner, C. Publication date 2008 Document Version Final published version Link to publication Citation for published version (APA): Wallner, C. (2008). Development of the pelvic floor : implications for clinical anatomy. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:01 Oct 2021 CHAPTER 3 THE INNERVATION OF THE PELVIC FLOOR MUSCLES: A REAPPRAISAL FOR THE LEVATOR ANI NERVE Published as: Wallner C, Maas CP, Dabhoiwala NF, Lamers WH, DeRuiter MC (2006). The Innervation of the Pelvic Floor Muscles: A Reappraisal for the Levator Ani Nerve. Obstet Gynecol, 108(3): 529-534. 75 CHAPTER 3 - INNERVATION OF THE PELVIC FLOOR MUSCLES: THE LEVATOR ANI NERVE Abstract Objective: A recent study, published in this journal suggested that a transvaginal pudendal nerve blockade selectively affects the pudendal nerve without blocking the direct sacral branches to the levator ani muscle (the levator ani nerve, LAN). -

An Introduction to Pelvic Floor Physical Therapy
Anatomy of the Pelvis Presented by Julie Peterson, PT, DPT, WCS, BCB- PMD Acknowledgements: Ruth M. Maher, PT, PhD, DPT, WCS, BCB-PMD October 2019 • Material presented in this webinar cannot be reproduced without consent of original authors. 2 Objectives • Understand pelvic anatomy – male/female – Osteology – Ligamentous – Viscera – Blood supply/lymphatics – Musculature/fascia – Innervation – lumbo sacral plexus, branches and structures innervated 3 The Politics of the Pelvis 4 Who’s looking after this system? Osteology 6 Pelvic (Hip) Girdle • Each coxal (hip) bone consists of three bones that fuse together: ilium, pubis, and ischium. • The two coxal bones are joined anteriorly by the pubic symphysis (fibrocartilage). • Joined posteriorly by the sacrum forming the sacroiliac joint (SIJ). • The os coxa (hip bone) initially begins life as three individual bones: – Ilium – Ischium – Pubis The Ilium • Largest of the three hip bones • Ilium is the superior part of the hip bone • Consists of a superior ala and inferior body which forms the acetabulum (the socket for the head of the femur) • Superior border - iliac crest • Greater sciatic notch - allows passage of sciatic nerve Ischium and Pubis • Ischium - inferior and posterior part of the hip bone • Most prominent feature is the ischial tuberosity, it is the part that meets the chair when you are sitting • Pubis - inferior and anterior part of the hip bone • Superior and inferior rami and body Anterior Pelvis Posterior Pelvis Superior View False and True Pelves • Pelvic brim/inlet - a line -

A Neglected Cause of Pain and Pelvic Floor Dysfunction Workshop Chair: Nucelio Lemos, Canada 13 September 2017 09:00 - 10:30
W24: Pudendal Neuralgia and Other Intrapelvic Peripheralnerve Entrapment- A Neglected Cause of Pain and Pelvic Floor Dysfunction Workshop Chair: Nucelio Lemos, Canada 13 September 2017 09:00 - 10:30 Start End Topic Speakers 09:00 09:15 Pelvic Neuroanatomy and Neurophysiology Nucelio Lemos 09:15 09:45 Peripheral Nerve Entrapment – From Diagnosis to Surgical Nucelio Lemos Treatment 09:45 10:00 Role, Techniques and Rationale of Physical Therapy on the Marilia Frare Post-Operative Treatment of Intrapelvic Nerve Entrapments 10:00 10:15 Musculoskeletal Nerve Entrapments and Myofascial Pain- The Nelly Faghani Role of Physical 10:15 10:30 Discussion and Wrap Up Nucelio Lemos, Marilia Frare, Nelly Faghani Speaker Powerpoint Slides Please note that where authorised by the speaker all PowerPoint slides presented at the workshop will be made available after the meeting via the ICS website www.ics.org/2017/programme Please do not film or photograph the slides during the workshop as this is distracting for the speakers. Aims of Workshop This workshop is directed to both clinicians and basic scientists interested in understanding the pathophysiology, clinical features and the therapeutic options of pudendal neuralgia and other intrapelvic nerve entrapments. The program starts with a review of the normal pelvic neuroanatomy through real surgery laparoscopic dissections. After this introduction, the clinical features of nerve entrapment syndromes will be explained, medical treatment guidelines will be proposed and the surgical treatment will be demonstrated by means of real surgery videos. The role of pelvic floor muscles in the etiopathogenesis of pelvic and perineal pain role of physical therapy will also be thoroughly discussed. -

Innervation of the Levator Ani and Coccygeus Muscles of the Female Rat
THE ANATOMICAL RECORD PART A 275A:1031–1041 (2003) Innervation of the Levator Ani and Coccygeus Muscles of the Female Rat RONALD E. BREMER,1 MATTHEW D. BARBER,2 KIMBERLY W. COATES,3 1,4 1,4,5 PAUL C. DOLBER, AND KARL B. THOR * 1Research Services, Veterans Affairs Medical Center, Durham, North Carolina 2Department of Obstetrics and Gynecology, Cleveland Clinic Foundation, Cleveland, Ohio 3Department of Obstetrics and Gynecology, Scott and White Clinic, Temple, Texas 4Department of Surgery, Duke University Medical Center, Durham, North Carolina 5Dynogen Pharmaceuticals, Inc., Durham, North Carolina ABSTRACT In humans, the pelvic floor skeletal muscles support the viscera. Damage to innervation of these muscles during parturition may contribute to pelvic organ prolapse and urinary incontinence. Unfortunately, animal models that are suitable for studying parturition-in- duced pelvic floor neuropathy and its treatment are rare. The present study describes the intrapelvic skeletal muscles (i.e., the iliocaudalis, pubocaudalis, and coccygeus) and their innervation in the rat to assess its usefulness as a model for studies of pelvic floor nerve damage and repair. Dissection of rat intrapelvic skeletal muscles demonstrated a general similarity with human pelvic floor muscles. Innervation of the iliocaudalis and pubocaudalis muscles (which together constitute the levator ani muscles) was provided by a nerve (the “levator ani nerve”) that entered the pelvic cavity alongside the pelvic nerve, and then branched and penetrated the ventromedial (i.e., intrapelvic) surface of these muscles. Inner- vation of the rat coccygeus muscle (the “coccygeal nerve”) was derived from two adjacent branches of the L6-S1 trunk that penetrated the muscle on its rostral edge. -

The Clinical Effects of Neuromodulation Therapies in the Treatment of Faecal Incontinence
MD(Res) Thesis 2016 THE CLINICAL EFFECTS OF NEUROMODULATION THERAPIES IN THE TREATMENT OF FAECAL INCONTINENCE __________________________________________________________________________________ 1 MD(Res) Thesis 2016 by Noel N. K. S. Thin Queen Mary’s National Centre for Bowel Research and Surgical Innovation A Thesis submitted for the Degree of Medical Doctorate (Research) The University of London November 2016 __________________________________________________________________________________ 2 MD(Res) Thesis 2016 ABSTRACT Background and Aims Sacral nerve stimulation (SNS) is an established therapy for faecal incontinence (FI). Percutaneous tibial nerve stimulation (PTNS) is a newer, less-invasive treatment. The effectiveness, cost and acceptability of these treatments have not been systematically compared. Methods A systematic review of neuromodulation interventions for FI and an investigator-blinded, randomised pilot trial of PTNS vs. SNS including parallel quantitative (clinical outcomes and cost) and qualitative studies. Results The systematic review determined on intention-to-treat, the median success rates for SNS were 63% (range 33-66%), 58% (range 52-81%) and 54% (range 50-58%) in the short, medium and long terms respectively. The success rate for PTNS was 59% at 12 months. In the pilot trial: 40 patients (39 female; mean age 59 years) met eligibility criteria. As designed, 23 were randomised to receive SNS and 17 PTNS. 15 patients progressed to permanent SNS implantation and 16 patients received a full course of PTNS. Within group effect sizes were marginally greater for SNS than PTNS on available case analysis. FI episodes per week at baseline, 3 months and 6 months follow-up: SNS median 5.75 (IQR 5.75-15.5 ) [mean 11.4 (SD 12.0)], 2.5 (2-4.5) [4.0 (4.0)], 1.75 (1.5-5) [4.9 (6.9)], vs. -

Three-Component Model of the Spinal Nerve Branching Pattern, Based on the View of the Lateral Somitic Frontier and Experimental Validation
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.29.227710; this version posted July 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Three-Component Model of the Spinal Nerve Branching Pattern, based on the View of the Lateral Somitic Frontier and Experimental Validation Shunsaku Homma1*, Takako Shimada1, Ikuo Wada2, Katsuji Kumaki3, Noboru Sato3, and Hiroyuki Yaginuma1 1 Department of Neuroanatomy and Embryology, 2 Department of Cell Science, Institute of Biomedical Sciences, Fukushima Medical University, 1 Hikarigaoka, Fukushima, 960-1295 JAPAN 3 Division of Gross Anatomy and Morphogenesis, Niigata University Graduate School of Medical and Dental Sciences, Niigata, 951-8510 JAPAN * Corresponding author: S. Homma, [email protected]. bioRxiv preprint doi: https://doi.org/10.1101/2020.07.29.227710; this version posted July 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT One of the decisive questions about human gross anatomy is unmatching the adult branching pattern of the spinal nerve to the embryonic lineages of the peripheral target muscles. The two principal branches in the adult anatomy, the dorsal and ventral rami of the spinal nerve, innervate the intrinsic back muscles (epaxial muscles), as well as the body wall and appendicular muscles (hypaxial muscles), respectively. However, progenitors from the dorsomedial myotome develop into the back and proximal body wall muscles (primaxial muscles) within the sclerotome-derived connective tissue environment. -

Chronic Female Pelvic Pain—Part 1: Clinical Pathoanatomy and Examination of the Pelvic Region
TUTORIAL Chronic Female Pelvic Pain—Part 1: Clinical Pathoanatomy and Examination of the Pelvic Region Gail Apte, PT, ScD*; Patricia Nelson, PT, ScD†; Jean-Michel Brisme´e, PT, ScD*; Gregory Dedrick, PT, ScD*; Rafael Justiz III, MD‡; Phillip S. Sizer Jr., PT, PhD* *Center for Rehabilitation Research, Texas Tech University Health Science Center, Lubbock, Texas; †Department of Physical Therapy, Eastern Washington University, Spokane, Washington; ‡Saint Anthony Pain Management, Oklahoma City, Oklahoma, U.S.A. n Abstract: Chronic pelvic pain is defined as the presence symptoms (lumbosacral, coccygeal, sacroiliac, pelvic floor, of pain in the pelvic girdle region for over a 6-month per- groin or abdominal region) can be followed to establish a iod and can arise from the gynecologic, urologic, gastro- basis for managing the specific pain generator(s) and man- intestinal, and musculoskeletal systems. As 15% of women age tissue dysfunction. n experience pelvic pain at some time in their lives with yearly direct medical costs estimated at $2.8 billion, effective eval- Key Words: myofacial pain, pelvic pain, signs and symp- uation and management strategies of this condition are toms, female examination necessary. This merits a thorough discussion of a systematic approach to the evaluation of chronic pelvic pain condi- tions, including a careful history-taking and clinical exami- INTRODUCTION nation. The challenge of accurately diagnosing chronic pelvic pain resides in the degree of peripheral and central Pain in the pelvic region can arise from musculoskele- sensitization of the nervous system associated with the tal, gynecologic, urologic, gastrointestinal, and/or neu- chronicity of the symptoms, as well as the potential influ- rological conditions.