Plasmodium Falciparum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DIRLIST6 01050000 01300000.Pdf
Signatory ID Name CIN Company Name 01050011 KALRA SUNITA U74899DL1967PTC004762 R K INTERNATIOONAL PRIVATE 01050016 GUPTA VIVEK U51109OR2006PTC009068 MAHAKASH RENEWABLES (INDIA) 01050022 BHANDARI PARAMBIR SINGH U51909DL1999PTC100363 AKILA OVERSEAS PRIVATE LIMITED 01050036 BHUPENDRA GUPTA U70100MH1995PTC086049 SUNDER BUILDERS AND 01050064 KIRITKUMAR MERCHANT SHISHIR U51900MH2000PTC127408 HANS D TO R SOLUTIONS PRIVATE 01050071 AGARWAL BINDU U45201WB1997PTC084989 PRINCE SAGAR KUTIR PRIVATE 01050072 BIJOY HARIPRIYA JAIN U70109MH2008PTC180213 SAAT RASTA PROPERTIES PRIVATE 01050072 BIJOY HARIPRIYA JAIN U01403MH2008PTC182992 GREEN VALLEY AGRICULTURE 01050082 JAI KARUNADEVI PRITHVIRAJ U36993KA1999PTC025485 RODEO DRIVE LUXURY PRODUCTS 01050126 DEEPCHAND JAIN PRITHVIRAJ U36993KA1999PTC025485 RODEO DRIVE LUXURY PRODUCTS 01050174 JOGINDER SANDHU SINGH U67120CH2004PTC027291 JAGUAR CONSULTANTS PRIVATE 01050220 NARAYANAMURTHY U15421TN2006PLC060417 BHIMAAS SUGARS AND CHEMICALS 01050224 JITENDRA MEHTA U51109TN2007PTC062423 MOOLRAJ VYAPAR PRIVATE 01050251 PRAKASH SRIVASTAVA U72300DL2007PTC160451 PRODIGII ECALL PRIVATE LIMITED 01050251 PRAKASH SRIVASTAVA U63040DL2008PTC180031 REACHING WILD LIFE TOURISM 01050257 LALITKUMAR MERCHANT URMIL U51900MH2000PTC127408 HANS D TO R SOLUTIONS PRIVATE 01050273 KUSUM MISHRA U29248UP1999PTC024344 MAXWELL GEARS PRIVATE LIMITED 01050286 DUGGAL PRINCE U70109DL2006PTC153384 M R BUILDWELL PRIVATE LIMITED 01050290 JAI MISHRA SHANKAR U29248UP1999PTC024344 MAXWELL GEARS PRIVATE LIMITED 01050309 JAIN MUKESH U00000DL1992PTC050812 -
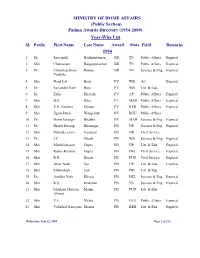
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Vol 116 No 1170; ISSN 1175 8716 Page 1 of 3 ©NZMA
THE NEW ZEALAND MEDICAL JOURNAL Journal of the New Zealand Medical Association CONTENTS 14 March 2003 This Issue in the Journal A summary of the original articles featured in this issue of the NZMJ Editorials The re-emergence of iodine deficiency in New Zealand? Jim Mann, Elizabeth Aitken Strike action by senior medical staff in Timaru – how did this come about? John Rietveld Small abdominal aortic aneurysms: exclusion or observation? Tim Buckenham Original Articles Mapping the themes of Maori talk about health Fiona Cram, Linda Smith, Wayne Johnstone Patients’ perception of the adequacy of informed consent: a pilot study of elective general surgical patients in Auckland Mikayla McKeague, John Windsor Symptom complaints following aerial spraying with biological insecticide Foray 48B Keith Petrie, Mark Thomas, Elizabeth Broadbent General practitioners’ perceptions of the nurse practitioner role: an exploratory study Bev Mackay Review Article The Queenstown Report: proposals for change in the medical disciplinary complaints process Wayne Cunningham, Murray Tilyard Viewpoints The sorry saga of the statins in New Zealand - pharmacopolitics versus patient care Evan Begg, Andrew Sidwell, Sharon Gardiner, Gary Nicholls, Russell Scott Response from PHARMAC: difficult choices Peter Moodie, Scott Metcalfe, Wayne McNee NZMJ 14 March 2003, Vol 116 No 1170; ISSN 1175 8716 Page 1 of 3 ©NZMA PHARMAC measures savings elsewhere to the health sector Scott Metcalfe, Sean Dougherty, Matthew Brougham, Peter Moodie Case Notes Takayasu’s arteritis and ulcerative -

DIN Name CIN Company Name 01050011 KALRA SUNITA U74899DL1967PTC004762 R K INTERNATIOONAL PRIVATE 01050016 GUPTA VIVEK U51109OR20
DIN Name CIN Company Name 01050011 KALRA SUNITA U74899DL1967PTC004762 R K INTERNATIOONAL PRIVATE 01050016 GUPTA VIVEK U51109OR2006PTC009068 MAHAKASH RENEWABLES (INDIA) 01050022 BHANDARI PARAMBIR SINGH U51909DL1999PTC100363 AKILA OVERSEAS PRIVATE LIMITED 01050036 BHUPENDRA GUPTA U65990MH1991PTC059930 GALAXY ESTATE AND 01050036 BHUPENDRA GUPTA U70100MH1995PTC086049 SUNDER BUILDERS AND 01050064 KIRITKUMAR MERCHANT SHISHIR U51900MH2000PTC127408 HANS D TO R SOLUTIONS PRIVATE 01050071 AGARWAL BINDU U45201WB1997PTC084989 PRINCE SAGAR KUTIR PRIVATE 01050072 BIJOY HARIPRIYA JAIN U01403MH2008PTC182992 GREEN VALLEY AGRICULTURE 01050072 BIJOY HARIPRIYA JAIN U70109MH2008PTC180213 SAAT RASTA PROPERTIES PRIVATE 01050082 JAI KARUNADEVI PRITHVIRAJ U36993KA1999PTC025485 RODEO DRIVE LUXURY PRODUCTS 01050126 DEEPCHAND JAIN PRITHVIRAJ U36993KA1999PTC025485 RODEO DRIVE LUXURY PRODUCTS 01050174 JOGINDER SANDHU SINGH U67120CH2004PTC027291 JAGUAR CONSULTANTS PRIVATE 01050177 RAJESH VERMA U24232DL1999PTC100334 S K MEDICOS PVT LTD 01050220 NARAYANAMURTHY U15421TN2006PLC060417 BHIMAAS SUGARS AND CHEMICALS 01050224 JITENDRA MEHTA U51109TN2007PTC062423 MOOLRAJ VYAPAR PRIVATE 01050227 KALRA RAMESH U74899DL1967PTC004762 R K INTERNATIOONAL PRIVATE 01050251 PRAKASH SRIVASTAVA U72300DL2007PTC160451 ProDigii ECall Private Limited 01050251 PRAKASH SRIVASTAVA U63040DL2008PTC180031 Reaching Wild Life Tourism Services 01050252 JADHAV RAJAN SHANKAR U55101PN2004PTC018986 HOTEL PUSHKAR GROUP PRIVATE 01050257 LALITKUMAR MERCHANT URMIL U51900MH2000PTC127408 HANS D TO R SOLUTIONS -

Zone Wise List of NASI Fellows
The National Academy of Sciences, India (The Oldest Science Academy of India) Zone wise list of Fellows & Honorary Fellows (2021) 5, Lajpatrai Road, Prayagraj – 211002, UP, India 1 The list has been divided into six zones; and each zone is further having the list of scientists of Physical Sciences and Biological Sciences, separately. 2 The National Academy of Sciences, India 5, Lajpatrai Road, Prayagraj – 211002, UP, India Zone wise list of Fellows Zone 1 (Bihar, Jharkhand, Odisha, West Bengal, Meghalaya, Assam, Mizoram, Nagaland, Arunachal Pradesh, Tripura, Manipur and Sikkim) (Section A – Physical Sciences) ACHARYA, Damodar, Chairman, Advisory Board, SOA Deemed to be University, Khandagiri Squre, Bhubanesware - 751030; ACHARYYA, Subhrangsu Kanta, Emeritus Scientist (CSIR), 15, Dr. Sarat Banerjee Road, Kolkata - 700029; ADHIKARI, Satrajit, Sr. Professor of Theoretical Chemistry, School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja SC Mullick Road, Jadavpur, Kolkata - 700032; ADHIKARI, Sukumar Das, Formerly Professor I, HRI,Ald; Professor & Head, Department of Mathematics, Ramakrishna Mission Vivekananda University, Belur Math, Dist Howrah - 711202; BAISNAB, Abhoy Pada, Formerly Professor of Mathematics, Burdwan Univ.; K-3/6, Karunamayee Estate, Salt Lake, Sector II, Kolkata - 700091; BANDYOPADHYAY, Sanghamitra, Professor & Director, Indian Statistical Institute, 203, BT Road, Kolkata - 700108; BANERJEA, Debabrata, Formerly Sir Rashbehary Ghose Professor of Chemistry,CU; Flat A-4/6,Iswar Chandra Nibas 68/1, Bagmari Road, Kolkata - 700054; BANERJEE, Rabin, Emeritus Professor, SN Bose National Centre for Basic Sciences, Block - JD, Sector - III, Salt Lake, Kolkata - 700098; BANERJEE, Soumitro, Professor, Department of Physical Sciences, Indian Institute of Science Education & Research, Mohanpur Campus, WB 741246; BANERJI, Krishna Dulal, Formerly Professor & Head, Chemistry Department, Flat No.C-2,Ramoni Apartments, A/6, P.G. -

Annual Report 2010/11
Annual Report 2010/11 NURTURING HUMAN CAPITAL FOR A HEALTHY WORLD AnnualTable Report of2010/11 Contents Sl. No. Particulars Page No. 1. Acknowledgement 11 2. Preamble 13 3. Chancellor’s Message 15 4. Pro-Chancellor’s Profile 16 5. Vice-Chancellor’s Profile & Message 17 6. Registrar’s Profile & Message 19 7. JSSU Organization Structure 21 8. Authorities of the University 23 9. Board of Management 23 10. Academic Council 24 11. Planning & Monitoring Board 26 12. Finance Committee 27 13. Advisory Committee 28 14. Hostel Review Committee 29 2 15. Library Committee 30 16. Anti-ragging Committee 31 17. Boards of Studies 33 18. Basic Medical Sciences (Post Graduate) 33 19. Medicine and Allied Subjects (Post Graduate) 33 20. Surgery & Allied Subjects (Post Graduate) 33 21. Pre & Para-clinical in the Faculty of Medicine (Under Graduate) 34 22. Clinical in the Faculty of Medicine (Under Graduate) 34 23. Dentistry (Post Graduate) 34 24. Dentistry (Under Graduate) 35 25. Pharmacy (Post Graduate) 35 26. Pharmacy (Under Graduate) 35 27. Research 36 28. Management Studies 36 29. Biomedical Sciences 36 30. Research Coordination Council 37 31. The Year in Review 2010-11 41 32. JSS University Administration 42 33. Academic Section 43 Annual Report 2010/11 34. Admission 43 35. Calendar of Events - Admissions 45 36. Fee Policy 46 37. Calendar of Events-Authorities & Boards of Studies 47 38. Distinguished & Adjunct Faculty 49 39. Collaborations 49 40. Examination Section 51 41. Overview of Results 53 42. JSS University PGET 2011-12 55 43. JSS University First Convocation 57 44. General Administration 65 45. -
Annual Report 2017-18
The National Academy of Sciences, India (NASI) Annual Report (April 2017 – March 2018) 5, Lajpatrai Road, Allahabad - 211 002 i POSTAL ADDRESS The National Academy of Sciences, India 5, Lajpatrai Road, Allahabad – 211002, India PHONE +91-532-2640224, 2441243 FAX +91-532-2641183 E-MAIL [email protected] WEBSITE http://www.nasi.nic.in http://www.nasi.org.in NASI, Allahabad is also on the FACEBOOK & TWITTER Published by the General Secretary (HQ), NASI for The National Academy of Sciences, India, Allahabad ii Late Prof. Meghnad Saha, Founder President An Academy of Science can do a great deal by educating public opinion, undertaking particular problems, and bringing out scientific workers in various fields for discussion and cooperative research. But the main function of the Academy should be towards cultural improvement by contributions to human knowledge. - Prof. Meghnad Saha on the Inaugural Session of the Academy India is justified in feeling proud for its unique contributions to science in ancient days. However, successive foreign invasions and alien rule for centuries pushed science in the background and the country went through with what may be described as the dark age for science. Western science attracted Indian intelligentsia after the establishment of the western system of education and the universities; and despite many constraints, the country could produce giants like Prof. Meghnad Saha, Prof. S. N. Bose, Sir J.C. Bose and Acharya Prafulla Chandra Ray. The first World War and the world-wide economic depression caused a set back to scientific research globally - much more so in India whose scientists found it difficult even to publish their research work since they had to be almost entirely dependent on foreign journals. -

Ciiknt Sknce
■// / ?50 y CIIKNT SKNCE Volume 118 Number 1 10 January 2020 * M-' ■ ' ■■■■uG •^'.,t»- ' I Jfi . ... ■• :■ ^.: .A- ivC" ^ > '' ■■ • •• •_ ii'^'v'; Pomegranate juice-fortified aonia candy Euglena population in a lake \ Normalising marks in multi-session examinations yd ■s"'' unfl. .' 'i Current Science Association Indian Academy of Sciences 9UB9CiUB£0 / central Libran' Founded: 1932 Stkkifii University ISSN: 0011-3891 NIIIHISCIHIN Volume 118 Number 1 10 January 2020 In this issue EDITORIAL Conservation of species, aii; big and small K. Chandrashekara CORRESPONDENCE 9 Congruence of endemism among four global biodiversity botspots in India V. S. Chitale, M. D. Behera and P. S. Roy 9 UGC-CARE List Bhushan Patwardhan and Shubhada Nagarkar; Response by V. Venkateswara Sarma NEWS 11 Science Last Fortnight 15 Precambrian geodynamics E. V. S. S. K. Babu, V. M. Tiwari and Somnath Dasgupta 17 Current challenges in the management of forest insect pests and diseases Shailesh Pandey, Sudhir Singh, Mohd. Yusuf and Amit Pandey 19 Indian Academy of Sciences, Bengaluru - 85th annual meeting Pratik Pawar and S. Priya COMMENTARY 22 Data and ideology of science Sumit Bhaduri SCIENTIFIC CORRESPONDENCE 24 Optimal Ph D student-teacher ratio for Indian research universities: a production function approach Pranab Mukhopadhyay, P. K. Sudarsan and M. P. Tapaswi 26 Influence of rake angle, bucket width and teeth depth of dragline bucket on the resistive force in different rock types Shah Fateh Azam and Piyush Rai TECHNICAL NOTE < 29 Improvement on the characteristics of transformer oil using nanofluids S. Sumathi and R. Rajesh GENERAL ARTICLES 34 Normalization of marks in multi-session examinations Abhay G. -

'Grand Challenges: India's Research Solutions to Real-World Problems'
India’s research solutions to real-world problems Biotechnology Industry Research Assistance Council We take our motto of “Ignite, innovate and incubate” seriously From funding, mentoring, supporting partnerships such as Grand Challenges India, and to assisting with IP matters, BIRAC has worked hard to create a support network for the budding biotech entrepreneurs of India. If you have a good idea and are looking for support, come apply for BIRAC schemes and Grand Challenges India. For more information, please contact: http://www.birac.nic.in/ | http://www.birac.nic.in/grandchallengesindia April 2018 EDITORIAL Editors: Subhra Priyadarshini, Rebecca Dargie FROM THE EDITOR Commentators: Govindarajan Padmanaban, Maharaj Kishan Bhan, Monkombu S Swaminathan, Renu Swarup, Shirshendu Mukherjee, Soumya Swaminathan, Trevor Mundel Art and design: Chandra Pal Singh ndia is headed towards an aston- What are the grand challenges for the Project management: Dalia El Essamy ishing population surge. With 1.34 country’s 1.3 billion people? Can sci- Photographic research: Madeline Hutchinson billion people recorded in early ence help find solutions to some of the 2018, the country is estimated public health problems? Can innova- PUBLISHING Ito add another 100 million by 2024 tion provide long-term answers? Nature Research Group overtaking China, currently the most Through in-depth commentaries ADVERTISING & SPONSORSHIP populous nation in the world. by subject experts, this special issue Institutional & Corporate Partnerships Therefore, her daunting demo- looks at the state of affairs in malaria Manager - India graphics are integral to any discussion management, maternal and child Sonia Sharma, M: +91 9650969959 [email protected] around the challenges faced by India. -

Department of Bio Technology
DEPARTMENT OF BIO TECHNOLOGY List of Ongoing Projects as on 08/07/2016 S.No. Title Project Investigator/Institute Date of Duration Total Cost Sanction (In Rs.) Agriculture Biotechnology – I 1 Programme Support for R&D in agricultural Prof. Anil Kumar Gupta 11/05/2012 5 Years 0 Month 27090000 Biotechnology-phase-II Programme at G.B. Pant G.B. Pant University of Agric. & Tech. University of Agriculture and Technology, Pant Pantnagar Pant Nagar, Uttarakhand- Nagar [BT/PR5031/AGR/2/850/2012] 263128 2 Program Support for Research and Development in Prof. R samiyappan 11/05/2012 5 Years 0 Month 31553004 Agricultural Biotechnology-Phase-II at Tamil Nadu Agricultural University TNAU,Coimbatore [BT/PR5095/AGR/2/847/2012] Coimbatore, Tamilnadu-641003 3 Characterization and molecular mapping of aphid Dr. Beant Singh 20/03/2015 3 Years 0 Month 5147600 (Rhopalosiphum maidis Fitch.) resistance in barley. Punjab Agricultural University [BT/PR9656/AGR/2/870/2013] Ludhiana, Punjab-141004 4 Emergence of Tobacco streak virus infecting Cotton Dr. Renukadevi P 20/04/2015 3 Years 0 Month 4970400 : Investigations on transmission, spread and Tamil Nadu Agricultural University symptom remission. Coimbatore, Tamilnadu-641003 [BT/PR11474/AGR/2/882/2014] 5 CHALLENGE PROGRAMME ON CHICKPEA Dr. Mukesh Jain 23/11/2015 5 Years 0 Month 78161600 FUNCTIONAL GENOMICS [BT/AGR/CG- National Institute of Plant Genome PhaseII/01/2014] Research New Delhi, Delhi-110067 Agriculture Biotechnology – II 6 Development of rice varieties for kerala with Dr. Jayalekshmy V G 02/08/2013 5 Years 0 Month 7240470 pyramided genes for Resistance to BLB by marker Kerala Agricultural University Thrissur, assisted selection [BT/PR13469/AGR/02/703/2010] Kerala-680656 7 Harnessing favorable QTL of wild and exotic Dr. -

Indian Council of Medical Research (ICMR) Is an Autonomous Organization Within the Union Minister of Health & Family Welfare, Government of India
The Indian Council of Medical Research (ICMR) is an autonomous organization within the Union Minister of Health & Family Welfare, Government of India. Established in 1911, REPORT it is the apex health research organization in the country. The mission of the ICMR is to promote better health in India through research. It provides stewardship, conducts and supports health research, generates knowledge and ensures its utilization and develops resources for health research in areas of national public health importance. Performance Appraisal Board The ICMR has evolved over the years in line with the changing health research needs. The role of research and modern science in fighting disease and improving health is being Indian Council of Medical Research increasingly re-emphasized. Links between research and disease control programmes are Indian Council of Medical Research being better appreciated. As a result of spectacular advances in modern science an array of new and emerging technologies have opened the doors to promising areas of research. They have changed the way we interpret information. The health challenges are also changing. The demands on ICMR are increasing too. Recognizing the need for a closely September 2005 defined and focused research strategy to meet the current and future challenges, the Union Ministry of Health & Family Welfare had constituted a Performance Appraisal Board in 2004, under chairmanship of Prof. K. Kasturirangan, Former Secretary, Department of Space & Chairman ISRO to review the ICMR. This document provides -

Govindarajan Padmanaban
PROFILE Govindarajan Padmanaban Many Indian scientists have been tempted by the allure of the “alienation” among segments of the population that had grown tired of West—but not G. Padmanaban. At the tail end of a career that the outdated portrayal of India as a land of poverty, dependence and filth. spans four decades, the biochemist has recently staked a more Padmanaban says many Westerners still have to be goaded into public presence, updating outdated notions of India and urging acknowledging India’s progress, but he remains well aware of the con- scientists to pursue a social mission. trasting mindsets of Indian scientists and their Western—particularly American—counterparts. Indian scientists, he says, have never had to work under much pressure to beat the competition and be the first to Early one Monday morning, during one of the 11 trips Govindarajan “reach the top.”But while he has long admired the American spirit of per- Padmanaban made to the University of Chicago between 1973 and severance and determination, he says, there is little else he encountered 1986, he overheard a colleague angrily inquiring about a batch of chem- that impressed him. “I believe you should enjoy what you are doing,”he icals that had been ordered the previous Friday and had not yet been says. “To be honest, I feel there are a lot of superficial things in the West, delivered. actually. People say,‘Oh, I enjoy my work’.I don’t think they do.” The conversation was just one more reminder of the vast difference Even as an emeritus professor, Padmanaban says, it is still the hours he between the American ethos and that of his native India, Padmanaban spends each day in his laboratory on the leafy campus of the IISc that give now says.“Here, I used to wait six months [for delivery of shipments],”he him the deepest pleasure.“When I see him working from 9 o’clock in the says, explaining that he never “got perturbed”or lost his temper in the lab.