Transformation of Riccia Fluitans, an Amphibious Liverwort Dynamically
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eleven Species of Liverworts As New Distributional Records to Bryoflora Andhra Pradesh, India
Bioscience Discovery, 11(3):111-120, July - 2020 © RUT Printer and Publisher Print & Online available on https://jbsd.in ISSN: 2229-3469 (Print); ISSN: 2231-024X (Online) Research Article Eleven species of liverworts as new distributional records to Bryoflora Andhra Pradesh, India. Ananthaneni Sreenath and Boyina Ravi Prasad Rao Biodiversity Conservation Division, Department of Botany, Sri Krishnadevaraya University, Ananthapuramu -515003, Andhra Pradesh. E-mail: [email protected] Article Info Abstract Received: 05-04-2020 Eleven species of liverworts viz., Asterella khasiana (Griff.) Grolle., Revised: 10-06-2020 Plagiochasma cordatum Lehm. & Lindenb., Plagiochasma intermedium Accepted: 18-06-2020 Lindend & Gottsche, Riccia frostii Austin, Riccia poihaniana A.E.D. Daniels Keywords: Eleven Species, & P. Daniel, Riccia sporocarpa Bisch. Riccia stricta (Gottsche, Lindenb. & Liverworts, New records, Nees) Perold, Riccia velimalaiana A.E.D. Daniels & P. Daniel, Andhra Pradesh Riccardia levieri Schiffner., Riccardia tenuicostata Schiffn. and Riccardia villosa (Stephani) S.C. Srivast. & Udar, are collected from different forest tracts of Eastern Ghats in Andhra Pradesh, are being new distributional records the state. INTRODUCTION: Plagiochasma intermedium Lindend & Gottsche, Andhra Pradesh is the seventh largest state Riccia frostii Austin, Riccia poihaniana A.E.D. in Indian union, it covers an area about 162, 970 sq. Daniels & P. Daniel, Riccia sporocarpa Bisch. kilometers and lies between 12°37ʹ and 19° 25ʹ Riccia stricta (Gottsche, Lindenb. & Nees) Perold, Northern latitude and 76° 45ʹ and 84° 72ʹ Eastern Riccia velimalaiana A.E.D. Daniels & P. Daniel, longitude (Map 1). The state comprises 13 districts, Riccardia levieri Schiffner., Riccardia tenuicostata there are two areas namely called Rayalaseema and Schiffn. and Riccardia villosa (Stephani) S.C. -

Phytotaxa, a Synthesis of Hornwort Diversity
Phytotaxa 9: 150–166 (2010) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2010 • Magnolia Press ISSN 1179-3163 (online edition) A synthesis of hornwort diversity: Patterns, causes and future work JUAN CARLOS VILLARREAL1 , D. CHRISTINE CARGILL2 , ANDERS HAGBORG3 , LARS SÖDERSTRÖM4 & KAREN SUE RENZAGLIA5 1Department of Ecology and Evolutionary Biology, University of Connecticut, 75 North Eagleville Road, Storrs, CT 06269; [email protected] 2Centre for Plant Biodiversity Research, Australian National Herbarium, Australian National Botanic Gardens, GPO Box 1777, Canberra. ACT 2601, Australia; [email protected] 3Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605-2496; [email protected] 4Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; [email protected] 5Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901; [email protected] Abstract Hornworts are the least species-rich bryophyte group, with around 200–250 species worldwide. Despite their low species numbers, hornworts represent a key group for understanding the evolution of plant form because the best–sampled current phylogenies place them as sister to the tracheophytes. Despite their low taxonomic diversity, the group has not been monographed worldwide. There are few well-documented hornwort floras for temperate or tropical areas. Moreover, no species level phylogenies or population studies are available for hornworts. Here we aim at filling some important gaps in hornwort biology and biodiversity. We provide estimates of hornwort species richness worldwide, identifying centers of diversity. We also present two examples of the impact of recent work in elucidating the composition and circumscription of the genera Megaceros and Nothoceros. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

From Southern Africa. 1. the Genus Dumortiera and D. Hirsuta; the Genus Lunularia and L
Bothalia 23,1: 4 9 -5 7 (1993) Studies in the Marchantiales (Hepaticae) from southern Africa. 1. The genus Dumortiera and D. hirsuta; the genus Lunularia and L. cruciata S.M. PEROLD* Keywords: Dumortiera, D. hirsuta, Dumortieroideae, Hepaticae, Lunularia, L cruciata. Lunulariaceae, Marchantiaceae, Marchantiales, taxonomy, southern Africa, Wiesnerellaceae ABSTRACT The genera Dumortiera (Dumortieroideae, Marchantiaceae) and Lunularia (Lunulariaceae), are briefly discussed. Each genus is represented in southern Africa by only one subcosmopolitan species, D. hirsuta (Swartz) Nees and L. cruciata (L.) Dum. ex Lindberg respectively. UITTREKSEL Die genusse Dumortiera (Dumortieroideae, Marchantiaceae) en Lunularia (Lunulariaceae) word kortliks bespreek. In suidelike Afrika word elke genus verteenwoordig deur slegs een halfkosmopolitiese spesie, D. hirsuta (Swartz) Nees en L. cruciata (L.) Dum. ex Lindberg onderskeidelik. DUMORTIERA Nees Monoicous or dioicous. Antheridia sunken in subses sile disciform receptacles, which are fringed with bristles Dumortiera Nees ab Esenbeck in Reinwardt, Blume and borne singly at apex of thallus on short bifurrowed & Nees ab Esenbeck, Hepaticae Javanicae, Nova Acta stalk. Archegonia in groups of 8—16 in saccate, fleshy Academiae Caesareae Leopoldina-Carolinae Germanicae involucres, on lower surface of 6—8-lobed disciform Naturae Curiosorum XII: 410 (1824); Gottsche et al.: 542 receptacle with marginal sinuses dorsally, raised on stalk (1846); Schiffner: 35 (1893); Stephani: 222 (1899); Sim: with two rhizoidal furrows; after fertilization and 25 (1926); Muller: 394 (1951-1958); S. Amell: 52 (1963); maturation, each involucre generally containing a single Hassel de Men^ndez: 182 (1963). Type species: Dumor sporophyte consisting of foot, seta and capsule; capsule tiera hirsuta (Swartz) Nees. wall unistratose, with annular thickenings, dehiscing irre gularly. -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

Ordovician Land Plants and Fungi from Douglas Dam, Tennessee
PROOF The Palaeobotanist 68(2019): 1–33 The Palaeobotanist 68(2019): xxx–xxx 0031–0174/2019 0031–0174/2019 Ordovician land plants and fungi from Douglas Dam, Tennessee GREGORY J. RETALLACK Department of Earth Sciences, University of Oregon, Eugene, OR 97403, USA. *Email: gregr@uoregon. edu (Received 09 September, 2019; revised version accepted 15 December, 2019) ABSTRACT The Palaeobotanist 68(1–2): Retallack GJ 2019. Ordovician land plants and fungi from Douglas Dam, Tennessee. The Palaeobotanist 68(1–2): xxx–xxx. 1–33. Ordovician land plants have long been suspected from indirect evidence of fossil spores, plant fragments, carbon isotopic studies, and paleosols, but now can be visualized from plant compressions in a Middle Ordovician (Darriwilian or 460 Ma) sinkhole at Douglas Dam, Tennessee, U. S. A. Five bryophyte clades and two fungal clades are represented: hornwort (Casterlorum crispum, new form genus and species), liverwort (Cestites mirabilis Caster & Brooks), balloonwort (Janegraya sibylla, new form genus and species), peat moss (Dollyphyton boucotii, new form genus and species), harsh moss (Edwardsiphyton ovatum, new form genus and species), endomycorrhiza (Palaeoglomus strotheri, new species) and lichen (Prototaxites honeggeri, new species). The Douglas Dam Lagerstätte is a benchmark assemblage of early plants and fungi on land. Ordovician plant diversity now supports the idea that life on land had increased terrestrial weathering to induce the Great Ordovician Biodiversification Event in the sea and latest Ordovician (Hirnantian) -

Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae
Glime, J. M. 2021. Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae. Chapt. 1-11. In: Glime, J. M. Bryophyte 1-11-1 Ecology. Volume 4. Habitat and Role. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 11 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-11: AQUATIC AND WET MARCHANTIOPHYTA, ORDER METZGERIALES: ANEURACEAE TABLE OF CONTENTS SUBCLASS METZGERIIDAE ........................................................................................................................................... 1-11-2 Order Metzgeriales............................................................................................................................................................... 1-11-2 Aneuraceae ................................................................................................................................................................... 1-11-2 Aneura .......................................................................................................................................................................... 1-11-2 Aneura maxima ............................................................................................................................................................ 1-11-2 Aneura mirabilis .......................................................................................................................................................... 1-11-7 Aneura pinguis .......................................................................................................................................................... -

Dumortier's Liverwort, Dumortiera Hirsuta (Sw.) Nees
Journal of the Arkansas Academy of Science Volume 73 Article 30 2019 Dumortier’s Liverwort, Dumortiera hirsuta (Sw.) Nees (Hepaticophyta: Marchantiales: Dumortieraceae) in Arkansas Chris T. McAllister Eastern Oklahoma St. College, [email protected] Henry W. Robison Retired, [email protected] Paul G. Davison University of North Alabama, [email protected] Follow this and additional works at: https://scholarworks.uark.edu/jaas Part of the Biology Commons, and the Other Forestry and Forest Sciences Commons Recommended Citation McAllister, Chris T.; Robison, Henry W.; and Davison, Paul G. (2019) "Dumortier’s Liverwort, Dumortiera hirsuta (Sw.) Nees (Hepaticophyta: Marchantiales: Dumortieraceae) in Arkansas," Journal of the Arkansas Academy of Science: Vol. 73 , Article 30. Available at: https://scholarworks.uark.edu/jaas/vol73/iss1/30 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. Dumortier’s Liverwort, Dumortiera hirsuta (Sw.) Nees (Hepaticophyta: Marchantiales: Dumortieraceae) in Arkansas Cover Page Footnote The Arkansas Game and Fish Commission (AG&F) and USDA, Ouachita National Forest, provided Scientific Collecting ermitsP to CTM and HWR. CTM thanks B. -
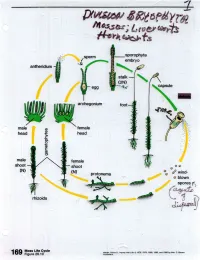
02-Bryophyta-2.Pdf
498 INTRODUCTORY PLANT SCIENCE layer. In certain species, the capsule con til1Ues to grow as long as the gametophyte lives. The presence of a meristcm in A11tlioceros and an aerating system complete with sto mata may indicate that Antlwceros evolved from ancestors with even ·larger· and more 1. Civ complex sporophytes. These features may Bryophyt be vestiges from a more complex ancestral. 2. Wh sporophyte. that ti alga ? 3. De� ORIGIN AND RELATIONSHIPS OF THE Bryophyt, BRYOPHYTA 4._ characteri Little is known with certainty about the r are small origin and evolution of the bryophytes. The ' tall. ( B) fossil record is too fragmenta to enable • ry s true roots us to trace their evolutionary history. Frag ,, sperms ar mentary remnants of thallose liverworts, spore idium and mother\4'/ttN) which resemble present-day liverworts, have cell Jar arch� been foum! in rocks of Carboniferous age, bryophyt as have structures that may be remains of more com mosses. all bryoph The immediate ancestors of the bryo bryo whicl phytes were probably more complex plants from the have an i than present-day forms. In other words, the gametophy evolutionary tendency has been one of re- with a dip1 duction instead of increased complexity. •• • • • spores If evolution has progressed . from more • . •, .... • s. __ complex sporophytes to those of simpler • . vascular p Anthoceros . form, the sporophyte of wou1d • • green algat be considered more ancient than that of • (D) red Marchantia or Riccia. Because Riccia has Fig. 35-16. longitudinal section of the spo 6. How the most reduced sporophyte, it would be t·:i;,hyte of Anthoceros. -

Biodiversity Profile of Afghanistan
NEPA Biodiversity Profile of Afghanistan An Output of the National Capacity Needs Self-Assessment for Global Environment Management (NCSA) for Afghanistan June 2008 United Nations Environment Programme Post-Conflict and Disaster Management Branch First published in Kabul in 2008 by the United Nations Environment Programme. Copyright © 2008, United Nations Environment Programme. This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. United Nations Environment Programme Darulaman Kabul, Afghanistan Tel: +93 (0)799 382 571 E-mail: [email protected] Web: http://www.unep.org DISCLAIMER The contents of this volume do not necessarily reflect the views of UNEP, or contributory organizations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP or contributory organizations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries. Unless otherwise credited, all the photos in this publication have been taken by the UNEP staff. Design and Layout: Rachel Dolores -

Creating Ponds for Rare Mosses and Liverworts
Creating ponds for rare mosses and liverworts Freshwater Habitats Trust 1. Mosses and liverworts Key messages Mosses and liverworts, collectively known as bryophytes, are an incredibly • Clean water is essential for diverse group of plants. There are over 1,000 species in the UK occurring all of our rarest mosses and in almost every habitat, from dappled shade in woodlands to almost bare liverworts. Avoid areas where limestone crags. Within these habitats the margins of ponds, lakes and the adjacent landuse could pools provide an important resource for many species because they add nutrients or pollution to provide areas of bare wet mud on which bryophytes can germinate surface waters or (Figure 1). groundwater. Unfortunately due to habitat loss, regulation of water levels and declines • On mineral or forestry sites, in the availability of clean unpolluted water, many bryophytes are now ensure that waterbodies have seriously threatened (Figure 2). By creating suitable pond habitats we can bryophyte friendly after uses. give rare mosses and liverworts the best chance of recovery. If needed, partition the site into areas for recreation and those for wildlife conservation. • Ensure that all waterbodies whether large or small have very wide shallow margins. Freshwater Habitats Trust This will increase the width of the drawdown zone and © Michael Lüth © Michael © David Holyoak © David Holyoak optimise the area available Figure 1. Bryophytes growing on bare mud within the pond margin: for bryophytes. Norfolk Bladder-moss Physcomitrium eurystomum (left) and Lizard • Create a complex of ponds of Crystalwort Riccia bifurca (right). different sizes. This will 2. Designing ponds for bryophytes provide a range of different environmental conditions and The spores of mosses and liverworts can readily move to new ponds on support the greatest number the feet of grazing animals and wildfowl. -

Liverworts, Mosses and Hornworts of Afghanistan - Our Present Knowledge
ISSN 2336-3193 Acta Mus. Siles. Sci. Natur., 68: 11-24, 2019 DOI: 10.2478/cszma-2019-0002 Published: online 1 July 2019, print July 2019 Liverworts, mosses and hornworts of Afghanistan - our present knowledge Harald Kürschner & Wolfgang Frey Liverworts, mosses and hornworts of Afghanistan ‒ our present knowledge. – Acta Mus. Siles. Sci. Natur., 68: 11-24, 2019. Abstract: A new bryophyte checklist for Afghanistan is presented, including all published records since the beginning of collection activities in 1839 ‒1840 by W. Griffith till present. Considering several unidentified collections in various herbaria, 23 new records for Afghanistan together with the collection data can be added to the flora. Beside a new genus, Asterella , the new records include Amblystegium serpens var. serpens, Brachythecium erythrorrhizon, Bryum dichotomum, B. elwendicum, B. pallens, B. weigelii, Dichodontium palustre, Didymodon luridus, D. tectorum, Distichium inclinatum, Entosthodon muhlenbergii, Hygroamblystegium fluviatile subsp. fluviatile, Oncophorus virens, Orthotrichum rupestre var. sturmii, Pogonatum urnigerum, Pseudocrossidium revolutum, Pterygoneurum ovatum, Schistidium rivulare, Syntrichia handelii, Tortella inflexa, T. tortuosa, and Tortula muralis subsp. obtusifolia . Therewith the number of species increase to 24 liverworts, 246 mosses and one hornwort. In addition, a historical overview of the country's exploration and a full biogeography of Afghan bryophytes is given. Key words: Bryophytes, checklist, flora, phytodiversity. Introduction Recording, documentation, identification and classification of organisms is a primary tool and essential step in plant sciences and ecology to obtain detailed knowledge on the flora of a country. In many countries, such as Afghanistan, however, our knowledge on plant diversity, function, interactions of species and number of species in ecosystems is very limited and far from being complete.