Phase-Field Modeling of Chemoelastic Binodal/Spinodal Relations and Solute Segregation to Defects in Binary Alloys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phase Diagrams and Phase Separation
Phase Diagrams and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon P Haasen, Physical Metallurgy, G Strobl, The Physics of Polymers, Springer Introduction Mixing two (or more) components together can lead to new properties: Metal alloys e.g. steel, bronze, brass…. Polymers e.g. rubber toughened systems. Can either get complete mixing on the atomic/molecular level, or phase separation. Phase Diagrams allow us to map out what happens under different conditions (specifically of concentration and temperature). Free Energy of Mixing Entropy of Mixing nA atoms of A nB atoms of B AM Donald 1 Phase Diagrams Total atoms N = nA + nB Then Smix = k ln W N! = k ln nA!nb! This can be rewritten in terms of concentrations of the two types of atoms: nA/N = cA nB/N = cB and using Stirling's approximation Smix = -Nk (cAln cA + cBln cB) / kN mix S AB0.5 This is a parabolic curve. There is always a positive entropy gain on mixing (note the logarithms are negative) – so that entropic considerations alone will lead to a homogeneous mixture. The infinite slope at cA=0 and 1 means that it is very hard to remove final few impurities from a mixture. AM Donald 2 Phase Diagrams This is the situation if no molecular interactions to lead to enthalpic contribution to the free energy (this corresponds to the athermal or ideal mixing case). Enthalpic Contribution Assume a coordination number Z. Within a mean field approximation there are 2 nAA bonds of A-A type = 1/2 NcAZcA = 1/2 NZcA nBB bonds of B-B type = 1/2 NcBZcB = 1/2 NZ(1- 2 cA) and nAB bonds of A-B type = NZcA(1-cA) where the factor 1/2 comes in to avoid double counting and cB = (1-cA). -

Interval Mathematics Applied to Critical Point Transitions
Revista de Matematica:´ Teor´ıa y Aplicaciones 2005 12(1 & 2) : 29–44 cimpa – ucr – ccss issn: 1409-2433 interval mathematics applied to critical point transitions Benito A. Stradi∗ Received/Recibido: 16 Feb 2004 Abstract The determination of critical points of mixtures is important for both practical and theoretical reasons in the modeling of phase behavior, especially at high pressure. The equations that describe the behavior of complex mixtures near critical points are highly nonlinear and with multiplicity of solutions to the critical point equations. Interval arithmetic can be used to reliably locate all the critical points of a given mixture. The method also verifies the nonexistence of a critical point if a mixture of a given composition does not have one. This study uses an interval Newton/Generalized Bisection algorithm that provides a mathematical and computational guarantee that all mixture critical points are located. The technique is illustrated using several ex- ample problems. These problems involve cubic equation of state models; however, the technique is general purpose and can be applied in connection with other nonlinear problems. Keywords: Critical Points, Interval Analysis, Computational Methods. Resumen La determinaci´onde puntos cr´ıticosde mezclas es importante tanto por razones pr´acticascomo te´oricasen el modelamiento del comportamiento de fases, especial- mente a presiones altas. Las ecuaciones que describen el comportamiento de mezclas complejas cerca del punto cr´ıticoson significativamente no lineales y con multipli- cidad de soluciones para las ecuaciones del punto cr´ıtico. Aritm´eticade intervalos puede ser usada para localizar con confianza todos los puntos cr´ıticosde una mezcla dada. -

Study of the LLE, VLE and VLLE of the Ternary System Water + 1-Butanol + Isoamyl Alcohol at 101.3 Kpa
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Submitted to Journal of Chemical & Engineering Data provided by Repositorio Institucional de la Universidad de Alicante This document is confidential and is proprietary to the American Chemical Society and its authors. Do not copy or disclose without written permission. If you have received this item in error, notify the sender and delete all copies. Study of the LLE, VLE and VLLE of the ternary system water + 1-butanol + isoamyl alcohol at 101.3 kPa Journal: Journal of Chemical & Engineering Data Manuscript ID je-2018-00308r.R3 Manuscript Type: Article Date Submitted by the Author: 27-Aug-2018 Complete List of Authors: Saquete, María Dolores; Universitat d'Alacant, Chemical Engineering Font, Alicia; Universitat d'Alacant, Chemical Engineering Garcia-Cano, Jorge; Universitat d'Alacant, Chemical Engineering Blasco, Inmaculada; Universitat d'Alacant, Chemical Engineering ACS Paragon Plus Environment Page 1 of 21 Submitted to Journal of Chemical & Engineering Data 1 2 3 Study of the LLE, VLE and VLLE of the ternary system water + 4 1-butanol + isoamyl alcohol at 101.3 kPa 5 6 María Dolores Saquete, Alicia Font, Jorge García-Cano* and Inmaculada Blasco. 7 8 University of Alicante, P.O. Box 99, E-03080 Alicante, Spain 9 10 Abstract 11 12 In this work it has been determined experimentally the liquidliquid equilibrium of the 13 water + 1butanol + isoamyl alcohol system at 303.15K and 313.15K. The UNIQUAC, 14 NRTL and UNIFAC models have been employed to correlate and predict LLE and 15 16 compare them with the experimental data. -

Chapter 9: Other Topics in Phase Equilibria
Chapter 9: Other Topics in Phase Equilibria This chapter deals with relations that derive in cases of equilibrium between combinations of two co-existing phases other than vapour and liquid, i.e., liquid-liquid, solid-liquid, and solid- vapour. Each of these phase equilibria may be employed to overcome difficulties encountered in purification processes that exploit the difference in the volatilities of the components of a mixture, i.e., by vapour-liquid equilibria. As with the case of vapour-liquid equilibria, the objective is to derive relations that connect the compositions of the two co-existing phases as functions of temperature and pressure. 9.1 Liquid-liquid Equilibria (LLE) 9.1.1 LLE Phase Diagrams Unlike gases which are miscible in all proportions at low pressures, liquid solutions (binary or higher order) often display partial immiscibility at least over certain range of temperature, and composition. If one attempts to form a solution within that certain composition range the system splits spontaneously into two liquid phases each comprising a solution of different composition. Thus, in such situations the equilibrium state of the system is two phases of a fixed composition corresponding to a temperature. The compositions of two such phases, however, change with temperature. This typical phase behavior of such binary liquid-liquid systems is depicted in fig. 9.1a. The closed curve represents the region where the system exists Fig. 9.1 Phase diagrams for a binary liquid system showing partial immiscibility in two phases, while outside it the state is a homogenous single liquid phase. Take for example, the point P (or Q). -

Definitions of Terms Relating to Phase Transitions of the Solid State
Definitions of terms relating to phase transitions of the solid state Citation for published version (APA): Clark, J. B., Hastie, J. W., Kihlborg, L. H. E., Metselaar, R., & Thackeray, M. M. (1994). Definitions of terms relating to phase transitions of the solid state. Pure and Applied Chemistry, 66(3), 577-594. https://doi.org/10.1351/pac199466030577 DOI: 10.1351/pac199466030577 Document status and date: Published: 01/01/1994 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Notes on Phase Seperation Chem 130A Fall 2002 Prof
Notes on Phase Seperation Chem 130A Fall 2002 Prof. Groves Consider the process: Pure 1 + Pure 2 Æ Mixture of 1 and 2 We have previously derived the entropy of mixing for this process from statistical principles and found it to be: ∆ =− − Smix kN11ln X kN 2 ln X 2 where N1 and N2 are the number of molecules of type 1 and 2, respectively. We use k, the Boltzmann constant, since N1 and N2 are number of molecules. We could alternatively use R, the gas constant, if we chose to represent the number of molecules in terms of moles. X1 and X2 represent the mole fraction (e.g. X1 = N1 / (N1+N2)) of 1 and 2, respectively. If there are interactions between the two ∆ components, there will be a non-zero Hmix that we must consider. We can derive an expression ∆H = 2γ ∆ ∆ for Hmix using H of swapping one molecule of type 1, in pure type 1, with one molecule of type 2, in pure type 2 (see adjacent figure). We define γ as half of this ∆H of interchange. It is a differential interaction energy that tells us the energy difference between 1:1 and 2:2 interactions and 1:2 interactions. By defining 2γ = ∆H, γ represents the energy change associated with one molecule (note that two were involved in the swap). ∆ γ Now, to calculate Hmix from , we first consider 1 molecule of type 1 in the mixed system. For the moment we need not be concerned with whether or not they actually will mix, we just want ∆ to calculate Hmix as if they mix thoroughly. -

1000 J Mole Μa ( ) = 4,0001.09 TJ Mole Μb
3.012 PS 6 THERMODYANMICS SOLUTIONS 3.012 Issued: 11.28.05 Fall 2005 Due: THERMODYNAMICS 1. Building a binary phase diagram. Given below are data for a binary system of two materials A and B. The two components form three phases: an ideal liquid solution, and two solid phases α and β that exhibit regular solution behavior. Use the given thermodynamic data to answer the questions below. LIQUID PHASE: ALPHA PHASE: L J " J µo = "500 " 4.8T µo = #6000 ( A ) mole ( A ) mole L J " J µo = "1,000 "10T µo = #1,000 ( B ) mole ( B ) mole ! ! (T is temperature in K) Ω = 8,368 J/mole ! ! BETA PHASE: " J µo = #4,000 #1.09T ( A ) mole " J µo = #13,552 # 2.09T ( B ) mole ! (T is temperature in K) ! Ω = 7,000 J/mole a. Plot the free energy of L, alpha, and beta phases (overlaid on one plot) vs. XB at the following temperatures: 2000K, 1500K, 1000K, 925K, and 800K. For each plot, denote common tangents and drop vertical lines below the plot to a ‘composition bar’, as illustrated in the example plot below. For each graph, mark the stable phases as a function of XB in the composition bar below the graph, as illustrated in the example. 3.012 PS 6 1 of 18 12/11/05 EXAMPLE: 3.012 PS 6 2 of 18 12/11/05 3.012 PS 6 3 of 18 12/11/05 b. Using the free energy curves prepared in part (a), construct a qualitatively correct phase diagram for this system in the temperature range 800K – 2000K. -
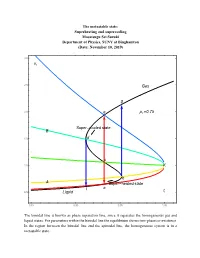
16.7 PT Superheating and Supercooling
The metastable state: Superheating and supercooling Masatsugu Sei Suzuki Department of Physics, SUNY at Binghamton (Date: November 10, 2019) 3.0 vr 2.5 Gas g 2.0 e pr 0.75 Super cooled state B 1.5 d c 1.0 K b A Super heated state l a 0.5 Liquid tr 0.85 0.90 0.95 1.00 The binodal line is known as phase separation line, since it separates the homogeneous gas and liquid states. For parameters within the binodal line the equilibrium shows two-phase co-existence. In the region between the binodal line and the spinodal line, the homogeneous system is in a metastable state. 3.0 vr 2.5 Gas p 0.70 r g pr 0.75 2.0 e pr 0.80 pr 0.85 B pr 0.90 1.5 d pr 0.95 c 1.0 K b A a l t 0.5 Liquid r 0.85 0.90 0.95 1.00 3.0 vr 2.5 Gas 2.0 g pr 0.80 e Super cooled state B 1.5 d c 1.0 K b A a l Super heated state 0.5 Liquid tr 0.85 0.90 0.95 1.00 e: the intersection of the ParametricPlot (denoted by black line) and the K-e line (binodal line) d: the intersection of the ParametricPlot (denoted by black line) and the K-d line (spinodal line) c: the intersection of the ParametricPlot (denoted by black line) and the K-c line b: the intersection of the ParametricPlot (denoted by black line) and the K-b line (spinodal line) a: the intersection of the ParametricPlot (denoted by black line) and the K-a line (binodal line) Point a: thermal equilibrium Point e: thermal equilibrium Point c: intermediate between the points a and b (temperature of point c is the same as that of points and e). -

Phase Equilibria of Quasi-Ternary Systems Consisting of Multicomponent Polymers in a Binary Solvent Mixture IV
Polymer Journal, Vol. 18, No.4, pp 347-360 (1986) Phase Equilibria of Quasi-Ternary Systems Consisting of Multicomponent Polymers in a Binary Solvent Mixture IV. Spinodal Curve and Critical Solution Point Kenji KAMIDE and Shigenobu MATSUDA Fundamental Research Laboratory of Fiber and Fiber-Forming Polymers, Asahi Chemical Industry Company, Ltd., 11-7, Hacchonawate, Takatsuki, Osaka 569, Japan (Received October 28, 1985) ABSTRACT: An attempt was made to construct a quasi-ternary system cons1stmg of multicomponent polymers dissolved in binary solvent mixture (solvents I and 2) to derive the equations of spinodal and neutral equilibrium conditions and to clarify the effects of three thermodynamic interaction x-parameters between solvent I and solvent 2, solvent I and polymer, and solvent 2 and polymer(x 12, x13, and x23) and the weight-average degree of polymerization and the ratio of to the number-average degree of polymerization of the original polymer on the spinodal curve and the critical points (the critical concentration calculated from the above mentioned equations. The cloud point curve was also calculated indirectly from coexisting curve evaluated according to a method in our previous papers and constant (starting polymer volume fraction) line. The cross point of a constant line and a coexisting curve is a cloud point. The cloud point curve thus calculated was confirmed to coincide, as theory predicted with the spinodal curve, at the critical point. decreases with an increase in X12, x23, and and increases with an increase in x13 -
![Arxiv:2108.08140V2 [Hep-Ph] 21 Aug 2021 Cal Potentials [21, 22]](https://docslib.b-cdn.net/cover/7864/arxiv-2108-08140v2-hep-ph-21-aug-2021-cal-potentials-21-22-2077864.webp)
Arxiv:2108.08140V2 [Hep-Ph] 21 Aug 2021 Cal Potentials [21, 22]
Critical point and Bose-Einstein condensation in pion matter V. A. Kuznietsov,1 O. S. Stashko,1 O. V. Savchuk,2 and M. I. Gorenstein2, 3 1Taras Shevchenko National University of Kyiv, 03022 Kyiv, Ukraine 2Frankfurt Institute for Advanced Studies Frankfurt am Main, Germany 3Bogolyubov Institute for Theoretical Physics, 03680 Kyiv, Ukraine (Dated: August 24, 2021) The Bose-Einstein condensation and the liquid-gas first order phase transition are studied in the interacting pion matter. Two phenomenological models are used: the mean-field model and the hybrid model. Free model parameters are fixed by fitting the lattice QCD data on the pion Bose condensate density at zero temperature. In spite of some minor differences the two model demonstrate an identical qualitative and very close quantitative behavior for the thermodynamic functions and electric charge fluctuations. A peculiar property of the considered models is an intersection of the Bose-Einstein condensation line and the line of the first order phase transition at the critical end point. Keywords: Bose-Einstein condensation, liquid-gas phase transition, pion matter I. INTRODUCTION and charged pions). In this specific region of the (µ, T ) phase diagram the QCD matter is expected in a form of the interacting pions. The heavier hadrons and/or quark- The Bose statistics [1] and Bose-Einstein condensation gluon degrees of freedom are expected to be suppressed (BEC) phenomenon [2] were predicted almost hundred at T m and not too large µ. years ago. The BEC can take place in equilibrium sys- Various approaches to a description of the pion matter tems of non-interacting bosons when a macroscopic part were developed: chiral perturbation theory [25, 26], lin- of all particles begins to occupy a single zero-momentum ear sigma model [27, 28], Nambu-Jona-Lasinio model [29] state. -

An Algebraic Geometric Method for Calculating Phase Equilibria from Fundamental Equations of State Hythem Sidky,1 Alan C
An algebraic geometric method for calculating phase equilibria from fundamental equations of state Hythem Sidky,1 Alan C. Liddell, Jr,2 Dhagash Mehta,1, 2 Jonathan D. Hauenstein,2, a) and Jonathan Whitmer1, b) 1)Department of Chemical and Biomolecular Engineering, University of Notre Dame, Notre Dame, IN 46556, USA. 2)Department of Applied and Computational Mathematics and Statistics, University of Notre Dame, Notre Dame, IN 46556, USA. Computing saturation properties from highly{accurate Helmholtz equations of state can be challenging for many reasons. The presence of multiple Maxwell loops often results in incorrect solutions to equations defining fluid phase coexistence. Near the critical point, the same equations also become ill{conditioned. As a consequence, without highly accurate initial guesses, it is difficult to avoid the trivial solution. Here, we propose an algorithm applying the technique of Newton homotopy continuation to determine the coexistence curve for all vapor{liquid equilibrium conditions between the triple point and critical point. Importantly, our algorithm is entirely convergence{parameter free, does not rely on the use of auxiliary equations, requires no initial guesses, and can be used arbitrarily close to the critical point. It is also fully generalizable to arbitrary equations of state, only requiring that they be locally analytic away from the critical point. We demonstrate that the method is capable of handling both technical and reference quality fundamental equations of state, is computationally inexpensive, and is useful in both evaluating individual state points and plotting entire fluid phase envelopes. Keywords: phase equilibrium, saturation conditions, Newton homotopy, phase equilibrium, nonlinear equa- tions, numerical algebraic geometry I. -

SOLUTIONS of EXERCISES 301 Central Role of Gibbs Energy 301:1
SOLUTIONS OF EXERCISES 301 central role of Gibbs energy 301:1 characteristic Numerical values in SI units: m.p = 2131.3; ΔS = 7.95; ΔH = 16943; ΔCP = −11.47. 301:2 a coexistence problem 1) f = M[T,P] – N[2 conditions] = 0 ; this excludes the first option; 2) by eliminating lnP from the two equilibrium equations in the ideal-gas approximation, the invariant point should follow from [60000 + 16 (T/K) = 0]; this excludes the second option. 301:3 trigonometric excess Gibbs energy – spinodal and binodal . -1 2 RTspin(X) = (4000 J mol )π X(1−X)sin2πX; limits at zero K are X = 0 (obviously) and X = 0.75; at 0.75 Tc the binodal mole fractions are about 0.11 and 0.52 301:4 from experimental equal-G curve to phase diagram ΔΩ = 1342 J.mol-1; Xsol = 0.635; Xliq = 0.435; see also van der Linde et al. 2002. 301:5 an elliptical stability field * The property ΔG A , which invariably is negative, passes through a maximum, when plotted against temperature. The maximum is reached for a temperature which is about ‘in the middle of the ellipse’. The property ΔΩ is positive. 301:6 a metastable form stabilized by mixing The stable diagram has three three-phase equilibria: αI + liquid + β at left; eutectic; liquid + β + αII at right; peritectic; αI + β + αII (below the metastable eutectic αI + liquid + αII); eutectoid. 301:7 the equal-G curve and the region of demixing For the left-hand case, where the EGC has a minimum, the stable phase diagram has a eutectic three-phase equilibrium.