Revised Biological Evaluation for the General NPDES Permit for Offshore
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTES on the BIRDS of CHIRIKOF ISLAND, ALASKA Jack J
NOTES ON THE BIRDS OF CHIRIKOF ISLAND, ALASKA JACK J. WITHROW, University of Alaska Museum, 907 Yukon Drive, Fairbanks, Alaska 99775; [email protected] ABSTRACT: Isolated in the western Gulf of Alaska 61 km from nearest land and 74 km southwest of the Kodiak archipelago, Chirikof Island has never seen a focused investigation of its avifauna. Annotated status and abundance for 89 species recorded during eight visits 2008–2014 presented here include eastern range extensions for three Beringian subspecies of the Pacific Wren (Troglodytes pacificus semidiensis), Song Sparrow (Melospiza melodia sanaka), and Gray-crowned Rosy-Finch (Leucost- icte tephrocotis griseonucha). A paucity of breeding bird species is thought to be a result of the long history of the presence of introduced cattle and introduced foxes (Vulpes lagopus), both of which persist to this day. Unique among sizable islands in southwestern Alaska, Chirikof Island (55° 50′ N 155° 37′ W) has escaped focused investigations of its avifauna, owing to its geographic isolation, lack of an all-weather anchorage, and absence of major seabird colonies. In contrast, nearly every other sizable island or group of islands in this region has been visited by biologists, and they or their data have added to the published literature on birds: the Aleutian Is- lands (Gibson and Byrd 2007), the Kodiak archipelago (Friedmann 1935), the Shumagin Islands (Bailey 1978), the Semidi Islands (Hatch and Hatch 1983a), the Sandman Reefs (Bailey and Faust 1980), and other, smaller islands off the Alaska Peninsula (Murie 1959, Bailey and Faust 1981, 1984). With the exception of most of the Kodiak archipelago these islands form part of the Alaska Maritime National Wildlife Refuge (AMNWR), and many of these publications are focused largely on seabirds. -

Wilderness in Southeastern Alaska: a History
Wilderness in Southeastern Alaska: A History John Sisk Today, Southeastern Alaska (Southeast) is well known remoteness make it wild in the most definitive sense. as a place of great scenic beauty, abundant wildlife and The Tongass encompasses 109 inventoried roadless fisheries, and coastal wilderness. Vast expanses of areas covering 9.6 million acres (3.9 million hectares), wild, generally undeveloped rainforest and productive and Congress has designated 5.8 million acres (2.3 coastal ecosystems are the foundation of the region’s million hectares) of wilderness in the nation’s largest abundance (Fig 1). To many Southeast Alaskans, (16.8 million acre [6.8 million hectare]) national forest wilderness means undisturbed fish and wildlife habitat, (U.S. Forest Service [USFS] 2003). which in turn translates into food, employment, and The Wilderness Act of 1964 provides a legal business. These wilderness values are realized in definition for wilderness. As an indicator of wild subsistence, sport and commercial fisheries, and many character, the act has ensured the preservation of facets of tourism and outdoor recreation. To Americans federal lands displaying wilderness qualities important more broadly, wilderness takes on a less utilitarian to recreation, science, ecosystem integrity, spiritual value and is often described in terms of its aesthetic or values, opportunities for solitude, and wildlife needs. spiritual significance. Section 2(c) of the Wilderness Act captures the essence of wilderness by identifying specific qualities that make it unique. The provisions suggest wilderness is an area or region characterized by the following conditions (USFS 2002): Section 2(c)(1) …generally appears to have been affected primarily by the forces of nature, with the imprint of man’s work substantially unnoticeable; Section 2(c)(2) …has outstanding opportunities for solitude or a primitive and unconfined type of recreation; Section 2(c)(3) …has at least five thousand acres of land or is of sufficient FIG 1. -

VGP) Version 2/5/2009
Vessel General Permit (VGP) Version 2/5/2009 United States Environmental Protection Agency (EPA) National Pollutant Discharge Elimination System (NPDES) VESSEL GENERAL PERMIT FOR DISCHARGES INCIDENTAL TO THE NORMAL OPERATION OF VESSELS (VGP) AUTHORIZATION TO DISCHARGE UNDER THE NATIONAL POLLUTANT DISCHARGE ELIMINATION SYSTEM In compliance with the provisions of the Clean Water Act (CWA), as amended (33 U.S.C. 1251 et seq.), any owner or operator of a vessel being operated in a capacity as a means of transportation who: • Is eligible for permit coverage under Part 1.2; • If required by Part 1.5.1, submits a complete and accurate Notice of Intent (NOI) is authorized to discharge in accordance with the requirements of this permit. General effluent limits for all eligible vessels are given in Part 2. Further vessel class or type specific requirements are given in Part 5 for select vessels and apply in addition to any general effluent limits in Part 2. Specific requirements that apply in individual States and Indian Country Lands are found in Part 6. Definitions of permit-specific terms used in this permit are provided in Appendix A. This permit becomes effective on December 19, 2008 for all jurisdictions except Alaska and Hawaii. This permit and the authorization to discharge expire at midnight, December 19, 2013 i Vessel General Permit (VGP) Version 2/5/2009 Signed and issued this 18th day of December, 2008 William K. Honker, Acting Director Robert W. Varney, Water Quality Protection Division, EPA Region Regional Administrator, EPA Region 1 6 Signed and issued this 18th day of December, 2008 Signed and issued this 18th day of December, Barbara A. -

Hubbard Glacier
National Park Service Park News U.S. Department of the Interior Hubbard Glacier Hubbard Glacier Fact Sheet --The Hubbard Glacier is North America’s largest tidewater glacier. It is 76 miles long, 7 miles wide, and 600 feet tall at its terminal face (350 feet exposed above the waterline and 250 feet below the waterline). --The Hubbard Glacier starts at Mt. Logan (19850 ft) in the Yukon Territory. Mt. Logan is the 2nd tallest mountain on the North American continent. --The Hubbard Glacier is currently advanc- ing (last 100 years), while most Alaskan glaciers are retreating (95%). This is not in contradiction with current global tempera- ture increases. The Hubbard Glacier will advance during times of warming climate and retreat in time of colder climates. The The Hubbard Glacier (right) and the Turner Glacier looking from Russell Fjord. current rate of advance is approximately 80 feet per year. 700 years ago – advanced to fill the entire pressure applied from the snow accumulat- --The glacier’s rate of overall forward ve- Yakutat Bay ing above. locity is much higher, but the advance is due its calving. Why do glaciers advance and retreat? --Once the ice becomes more than 150 feet thick the ice can behave plastically, and --The ice you see at the terminal face is ap- --Glaciers will always try to reach a balance start to flow under the influence of gravity. proximately 450 years old and is over 2000 between the amount of ice they gain to feet thick at some locations. the amount of ice they lose (equilibrium). -

Public Law 96-487 (ANILCA)
APPENDlX - ANILCA 587 94 STAT. 2418 PUBLIC LAW 96-487-DEC. 2, 1980 16 usc 1132 (2) Andreafsky Wilderness of approximately one million note. three hundred thousand acres as generally depicted on a map entitled "Yukon Delta National Wildlife Refuge" dated April 1980; 16 usc 1132 {3) Arctic Wildlife Refuge Wilderness of approximately note. eight million acres as generally depicted on a map entitled "ArcticNational Wildlife Refuge" dated August 1980; (4) 16 usc 1132 Becharof Wilderness of approximately four hundred note. thousand acres as generally depicted on a map entitled "BecharofNational Wildlife Refuge" dated July 1980; 16 usc 1132 (5) Innoko Wilderness of approximately one million two note. hundred and forty thousand acres as generally depicted on a map entitled "Innoko National Wildlife Refuge", dated October 1978; 16 usc 1132 (6} Izembek Wilderness of approximately three hundred note. thousand acres as �enerally depicted on a map entitied 16 usc 1132 "Izembek Wilderness , dated October 1978; note. (7) Kenai Wilderness of approximately one million three hundred and fifty thousand acres as generaJly depicted on a map entitled "KenaiNational Wildlife Refuge", dated October 16 usc 1132 1978; note. (8) Koyukuk Wilderness of approximately four hundred thousand acres as generally depicted on a map entitled "KoxukukNational Wildlife Refuge", dated July 1980; 16 usc 1132 (9) Nunivak Wilderness of approximately six hundred note. thousand acres as generally depicted on a map entitled "Yukon DeltaNational Wildlife Refuge", dated July 1980; 16 usc 1132 {10} Togiak Wilderness of approximately two million two note. hundred and seventy thousand acres as generally depicted on a map entitled "Togiak National Wildlife Refuge", dated July 16 usc 1132 1980; note. -

2018 Uncruise Adventures Brochure
October 2017 Adventure Cruises Define Your to April 2019 22 to 86 Guests Un-nessSM ALASKA | MEXICO | HAWAIIAN ISLANDS | COSTA RICA | PANAMÁ | GALÁPAGOS | COLUMBIA & SNAKE RIVERS | WASHINGTON | BRITISH COLUMBIA Contents Define Your Un-ness 3 Small Ships, BIG Adventures 5 Adventure 6 Place 8 Connection 10 Finding Our Un-ness 12 Unparalleled Value 14 Ready. Set. Go. 16 Theme Cruises 18 Wellness Cruises 20 Family Discoveries 22 Solo Travel 23 Groups & Charters 24 Sailing Calendar 26 COSTA RICA & PANAMÁ 28 MEXICO’S SEA OF CORTÉS 40 HAWAIIAN ISLANDS 48 GALÁPAGOS ISLANDS 56 COLUMBIA & SNAKE RIVERS 64 PACIFIC NORTHWEST 72 ALASKA 82 Life On Board 116 Wining & Dining 118 The Fleet 122 Small Ship Comparison 142 What’s Included 144 Reservation Information 145 Responsible Travel & Affiliations 146 Welcome Aboard 147 2 UnCruise.com Define Your Un-nessSM [uhn-nis] To break away from the masses. Challenge. Freely used to release, exemplify, or intensify a force or quality. To engage, connect, and explore unique places, oneself, and with others on a most uncommon adventure. Snapshot: I found my happy place. unique. 3 “Exceeded expectations. Thanks to the crew—you are fabulous. Only downside? My cheeks hurt from smiling. Awesome, fantastic!” -Nancy D; Silver Lake, NH (Alaska 2016) 4 UnCruise.com Snapshot: (L) Best to pack your Alaska tennis shoes. (R) Go with the flow. Small Ships, BIG Adventures A crew member shows you to your cabin. After a short time getting situated, gain your bearings with a spin around the ship. Then head to the lounge for a glass of bubbly and to meet your shipmates. -

Chuck River Wilderness Endicott River Wilderness Kootznoowoo
US DEPARTMENT OF AGRICULTURE FOREST SERVICE ALASKA REGION Klukwan Skagway TONGASS NATIONAL FOREST Ò 1:265,000 3 1.5 0 3 6 9 12 Miles 4.5 2.25 0 4.5 9 13.5 18 Kilometers [ Cities Congressionally Designated LUD II Areas and Monument Mainline Roads Wilderness/Monument Wilderness Other Road Canada Roaded Roadless National Park 2001 Roadless Areas National Wildlife Refuge Tongass 77 VCU AK Mental Health Trust Land Exchange Land Returned to NFS Non-Forest Service Land Selected by AK Mental Health Haines Development LUD* Tongass National Forest * Development LUDs include Timber Production, Modified Landscape, Scenic Viewshed, and Experimental Forest Map Disclaimer: The USDA Forest Service makes no warranty, expressed or implied, including the warranties of merchantability and fitness for a particular purpose, nor assumes any legal liability or responsibility for the accuracy, reliability, completeness or utility of these geospatial data, or for the improper or incorrect use of these geospatial data. These geospatial data and related maps or graphics are not legal documents and are not intended to be used as such. The data and maps may not be used to determine title, ownership, legal descriptions or boundaries, legal jurisdiction, or restrictions that may be in place on either public or private land. Natural hazards may or may not be depicted on the data and maps, and land users should exercise due caution. The data are dynamic and may change over time. The user is responsible to verify the limitations of the geospatial data and to use the data accordingly and use constraints information. Map 2/6 Endicott River Wilderness Gustavus Pleasant/Lemusurier/Inian Islands Wilderness Elfin Cove Pelican Hoonah Juneau West Chichagof-Yakobi Wilderness Tenakee Springs Kootznoowoo Wilderness Angoon Tracy Arm-Fords Terror Wilderness Chuck River Wilderness Sitka Sources: Esri, GEBCO, NOAA, National Geographic, Garmin, HERE, Geonames.org, and other contributors, Esri, Garmin, GEBCO, NOAA NGDC, and other contributors. -

A BILL to Designate Certain Lands As Wilderness
CONGRESS *>J"k<4 1STSESSXO* 3014 IN THE SENATE OF THE UNITED STATES OCTOBER 9,1969 Mr. JACKSON (for himself, Mr. ANDERSON, Mr. GRAVEL, Mr. MAGNUSON, Mr. MONTOYA, and Mr. STEVENS) introduced the following bill; which was read twice and referred to the Committee on Interior and Insular Affaira A BILL To designate certain lands as wilderness. 1 Be it enacted by the Senate and House of Representa- 2 lives of the United States of America in Congress assemble^ 3 That, (a) in accordance with section 3 (c) of the Wilderness 4 Act (78 Stat. 890; 16 U.S.C. 1132 (c) ), the following lands 5 are hereby designated as wilderness, and shall be adininis^ 6 tered by the Secretary of the Interior in accordance with th>$ 7 provisions of the Wilderness Act: : 8 (1) certain lands in the Hart Mountain Rational 9 Antelope Refuge, Oregon, which comprise about forty- 10 eight thousand acres and which are depicted on a map 11 entitled "Hart Mountain National Antelope, Refuge II 2 1 Wilderness—Proposed", dated August 1967, which shall 2 be known as the "Hart Mountain National Antelope 3 Refuge Wilderness"; 4 (2) certain lands in the Bering Sea, Bogoslof, and 5 Tuxedni National Wildlife Refuges, Alaska, as depicted ti on maps entitled "Bering Sea Wilderness—Proposed", 7 "Bogoslof Wilderness—Proposed", and "Tuxedni Wil- 8 derness—Proposed", dated August 1967, and the lands 9 comprising the St. Lazaria, Hazy Islands, and For- 10 rester Island National Wildlife Refuges, Alaska, as 11 depicted on maps entitled "Southeastern Alaska Pro- 12 posed Wilderness Areas", dated August 1967, which 13 shall be known as the "Bering Sea Wilderness", "Bogos- 14 lof Wilderness", "Tuxedni Wilderness", "St. -
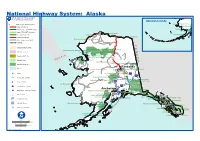
National Highway System: Alaska U.S
National Highway System: Alaska U.S. Department of Transportation Federal Highway Administration Aleutian Islands Eisenhower Interstate System Lake Clark National Preserve Lake Clark Wilderness Other NHS Routes Non-Interstate STRAHNET Route Katmai National Preserve Katmai Wilderness Major STRAHNET Connector Lonely Distant Early Warning Station Intermodal Connector Wainwright Dew Station Aniakchak National Preserve Barter Island Long Range Radar Site Unbuilt NHS Routes Other Roads (not on NHS) Point Lay Distant Early Warning Station Railroad CC Census Urbanized Areas AA Noatak Wilderness Gates of the Arctic National Park Cape Krusenstern National Monument NN Indian Reservation Noatak National Preserve Gates of the Arctic Wilderness Kobuk Valley National Park AA Department of Defense Kobuk Valley Wilderness AA D II Gates of the Arctic National Preserve 65 D SSSS UU A National Forest RR Bering Land Bridge National Preserve A Indian Mountain Research Site Yukon-Charley Rivers National Preserve National Park Service College Fairbanks Water Campion Air Force Station Fairbanks Fortymile Wild And Scenic River Fort Wainwright Fort Greely (Scheduled to close) Airport A2 4 Denali National Park A1 Intercity Bus Terminal Denali National PreserveDenali Wilderness Wrangell-Saint Elias National Park and Preserve Tatalina Long Range Radar Site Wrangell-Saint Elias National Preserve Ferry Terminal A4 Cape Romanzof Long Range Radar Site Truck/Pipeline Terminal A1 Anchorage 4 Wrangell-Saint Elias Wilderness Multipurpose Passenger Facility Sparrevohn Long -

Border Security Threatens Northern Border Wildernesses
Wilderness In Peril: Border Security Measures Threaten Wilderness along the Northern Border with Canada An Analysis Prepared by Wilderness Watch October 2012 Wilderness Watch P.O. Box 9175 Missoula, MT 59807 406-542-2048 www.wildernesswatch.org For more information, contact: George Nickas, Executive Director Kevin Proescholdt, Conservation Director [email protected] [email protected] 406-542-2048 612-201-9266 2 Table of Contents Executive Summary………………………………………………...…………….Page 3 Introduction………………………………………………………..………..….....Page 4 Background…………………………………………………..………………....…Page 4 A. Early 20th Century Border Easements B. International Boundary Treaties with Canada C. 2005 REAL ID Act D. 2006 Interagency Memorandum of Understanding (MOU) Border Patrol Practices on the Southern Border and Lessons for the North……………………………………………………………….Page 9 A. Border Wall Construction B. Illegal Roads and Vehicle Routes C. Border Security Infrastructure D. Motorized Patrols Emerging Major Threats to Wildernesses near the Northern Border……...…Page 13 A. Congressional Legislation B. Northern Border Programmatic Environmental Impact Statement C. 2006 MOU and Motorized Patrols D. Administrative Waiver of Federal Laws E. Clearing and Construction in Border Reservations F. Conclusion Needed Actions to Reestablish and Affirm Wilderness Protections Along the Northern Border……………………………………….……………..Page 17 A. Existing Homeland Security Laws B. 2006 MOU C. Northern Border PEIS D. Pending Legislation E. Restore Wilderness Protection Appendix - Wildernesses at Risk along the Northern Border………………....Page 18 3 Executive Summary Under the guise of border security, a plethora of new and proposed laws, policies, memoranda, and other governmental actions pose an unprecedented threat to Wildernesses, including in many national parks, along our nation’s Northern Border. This whitepaper describes the threats and presents several recommendations for securing the protection of Wilderness and parks along the Northern Border. -

Wilderness in the Circumpolar North
Planning in the Human Ecotone: Managing Wild Places on the Togiak National Wildlife Refuge Stewart Allen Abstract—The U.S. Fish and Wildlife Service is revising the long-range plan for Alaska’s Togiak National Wildlife Refuge, over half of which is designated as the Togiak Wilderness Change, meaningful change, Area. Many of the planning issues are social rather than biological, involving public use and almost always comes from its effects on Refuge resources and opportunities. Planners, managers, and stakeholders are the edge, the margin. finding themselves in the human ecotone, where two or more legal, social, and cultural edges Paul Hawken intersect to produce an abundance and diversity of conflicts, challenges, and opportunities. Planning in the human ecotone requires collaboration, up-to-date information, and an adap- tive process. Introduction _____________________________________________________ Located in remote southwest Alaska, the Togiak National Wildlife Refuge com- prises 4.3 million acres between Kuskokwim Bay and Bristol Bay. Congress estab- lished the Refuge in 1980 under the Alaska National Interest Lands Conservation Act, which not only greatly expanded an existing refuge but designated just over half of the new refuge as the Togiak Wilderness Area—the second largest Wilder- ness in the entire Refuge System (fig. 1). The Alaska National Interest Lands Con- servation Act directed the U.S. Fish and Wildlife Service to develop a long-range Comprehensive Conservation Plan for the area, which was published in 1987. In 2000, the U.S. Fish and Wildlife Service began revising the original plan, tak- ing into account new information, resource conditions, and public uses. -

Calendar No. 589 91St CONGRESS ) SENATE EXPORT 1St Session J No
Calendar No. 589 91sT CONGRESS ) SENATE EXPORT 1st Session J No. 91-504 HART MOUNTAIN NATIONA^ ANTELOPE REFUGE, ETC- DECEMBER 9, 1909.—Ordered to be printed Mr. JACKSON, from the Committee on Interior and Insular Affairs, submitted the following REPORT . [To accompany S. 3014] The Committee on. Interior and Insular A.ff airs',:; to. which,,was re- ferred the bill (S. 3014.)..to designate.certain ,lund.s.iu tl.ie IIa-r,t JMtoun-. tain National Antelope* Refuge, =the MalheurlKa^ipiiaji W.ijdiife Rpfr uge, the Three Arph.Rqc.ks and Oregon •Xs.la.n.ds National .Wildlife Refuges, all in Oregon; ..the-Bering .Sea,... ^ogqslpf, Tuxedrii,. ^t. Lazaria, Hazy Islands, ^nd Forrester IsJta,nd,,Jtfationa,l Wildlife Ref- uges, all hi Alaska; the Copalis, Flattery; Rbcljs.andQujll}»y#te Neer dies National ^ildlife Rpfug^in^he.^iatfl'pijiV^aslung^nj'wid the Bitter Lafce National Wildlife Refuge/in,.j^W ¥^exjipo, as wilUerness, having considered tlie same, renorts fayprably.;t)iereon with amend- ments and recommends that the bill as amen^1 ' ' PURPOSE , :•;•;•; .-.- • -i I . If! Tlxis >ill, S, .301*, iiiuced'>ypuld /wve^desighate.d as.\iuijts of the National Wilderness' 'reservation System, the Hart Mountain( National Antelope,Refu the. Malheuy Natioinal WildlifeJRefuge,' the Three Arch Ixpcks a«h Oregon Islands National ^•'n'1i>'^ " <--*'--1-"1 -*-1— -:'-- - «y- .in Wildlife Refuses in ther State..of jWasinn'gftni,'and. the.Biljter Lake. National Wildlife Refuge in New -Mexico. AJl of the.Uwids.are prep- ently within the National .Wildjife Refjige, System, and no land acquisition costs are involved. TJiege. wilderness.; prpposftls.