Inherited Patterned Lentiginosis: a Diagnosis of Exclusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Nevus Spilus: Is the Presence of Hair Associated with an Increased Risk for Melanoma?
Nevus Spilus: Is the Presence of Hair Associated With an Increased Risk for Melanoma? Robert Milton Gathings, MD; Raveena Reddy, MD; Ashish C. Bhatia, MD; Robert T. Brodell, MD PRACTICE POINTS • Nevus spilus (NS) appears as a café au lait macule studded with darker brown “moles.” • Although melanoma has been described in NS, it is rare. • There is no evidence that hairy NS are predisposed to melanoma. copy not Nevus spilus (NS), also known as speckled len- he term nevus spilus (NS), also known as tiginous nevus, is characterized by background speckled lentiginous nevus, was first used café au lait–like lentiginous melanocytic hyperpla- Tin the 19th century to describe lesions with sia speckled with small, 1- to 3-mm, darker foci.Do background café au lait–like lentiginous melanocytic Nevus spilus occurs in 1.3% to 2.3% of the adult hyperplasia speckled with small, 1- to 3-mm, darker population worldwide. Reports of melanoma aris- foci. The dark spots reflect lentigines; junctional, ing within hypertrichotic NS suggest that hyper- compound, and intradermal nevus cell nests; and trichosis may be a marker for the development of more rarely Spitz and blue nevi. Both macular and melanoma. We present a case of a hypertrichotic papular subtypes have been described.1 This birth- NS without melanoma and also provide a review of mark is quite common, occurring in 1.3% to 2.3% previously reported cases of hypertrichosis in NS. of the adult population worldwide.2 Hypertrichosis We believe that NS has aCUTIS lower risk for malignant has been described in NS.3-9 Two subsequent cases degeneration than congenital melanocytic nevi of malignant melanoma in hairy NS suggested that (CMN) of the same size, and it is unlikely that lesions may be particularly prone to malignant hypertrichosis is a marker for melanoma in NS. -

Short Course 11 Pigmented Lesions of the Skin
Rev Esp Patol 1999; Vol. 32, N~ 3: 447-453 © Prous Science, SA. © Sociedad Espafiola de Anatomfa Patol6gica Short Course 11 © Sociedad Espafiola de Citologia Pigmented lesions of the skin Chairperson F Contreras Spain Ca-chairpersons S McNutt USA and P McKee, USA. Problematic melanocytic nevi melanin pigment is often evident. Frequently, however, the lesion is solely intradermal when it may be confused with a fibrohistiocytic RH. McKee and F.R.C. Path tumor, particularly epithelloid cell fibrous histiocytoma (4). It is typi- cally composed of epitheliold nevus cells with abundant eosinophilic Brigham and Women’s Hospital, Harvard Medical School, Boston, cytoplasm and large, round, to oval vesicular nuclei containing pro- USA. minent eosinophilic nucleoli. Intranuclear cytoplasmic pseudoinclu- sions are common and mitotic figures are occasionally present. The nevus cells which are embedded in a dense, sclerotic connective tis- Whether the diagnosis of any particular nevus is problematic or not sue stroma, usually show maturation with depth. Less frequently the nevus is composed solely of spindle cells which may result in confu- depends upon a variety of factors, including the experience and enthusiasm of the pathologist, the nature of the specimen (shave vs. sion with atrophic fibrous histiocytoma. Desmoplastic nevus can be distinguished from epithelloid fibrous histiocytoma by its paucicellu- punch vs. excisional), the quality of the sections (and their staining), larity, absence of even a focal storiform growth pattern and SiQO pro- the hour of the day or day of the week in addition to the problems relating to the ever-increasing range of histological variants that we tein/HMB 45 expression. -
Diagnostic Pitfall of Localized Lentigo Accompanied by Post-Inflammatory
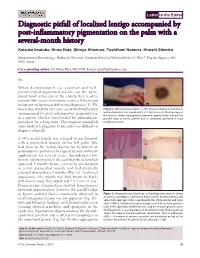
Letter to the Editor Diagnostic pitfall of localized lentigo accompanied by post-inflammatory pigmentation on the palm with a several-month history Keisuke Imafuku, Hiroo Hata, Shinya Kitamura, Toshifumi Nomura, Hiroshi Shimizu Department of Dermatology, Hokkaido University Graduate School of MedicineNorth 15, West 7, Kita-ku, Sapporo 060- 8638, Japan Corresponding author: Dr. Hiroo Hata, MD, PhD, E-mail: [email protected] Sir, When dermatologists see acquired and well- circumscribed pigmented macules on the palm, dorsal hand or forearm of the elderly, they tend to consider blue nevus, hematoma, nevus cell nevus and malignant melanoma as differential diagnoses [1]. We a b herein described the very rare case of localized lentigo Figure 1: Clinical manifestations. a. The macule is brown to dark black, accompanied by post-inflammatory pigmentation well-demarcated, flat, round and 3 x 4 mm in size. b. Dermoscopy of the macule shows homogenous brownish pigmentation without the in a patient who has been treated for palmoplanter parallel ridge or furrow pattern that is commonly observed in acral pustulosis for a long time. This eruption mimicked lentiginous lesion some kinds of lentiginous lesion and it was difficult to diagnose clinically. A 59-year-old female was referred to our hospital with a pigmented macule on her left palm. She had been to the family doctor for treatment of palmoplanter pustulosis by topical steroid ointment application for several years. According to the a history-taking interview, she said that the lesion had b appeared 5 months before referral to our hospital as a tiny pigmented macule and had gradually enlarged during those 5 months (Fig. -

Phacomatosis Spilorosea Versus Phacomatosis Melanorosea
Acta Dermatovenerologica 2021;30:27-30 Acta Dermatovenerol APA Alpina, Pannonica et Adriatica doi: 10.15570/actaapa.2021.6 Phacomatosis spilorosea versus phacomatosis melanorosea: a critical reappraisal of the worldwide literature with updated classification of phacomatosis pigmentovascularis Daniele Torchia1 ✉ 1Department of Dermatology, James Paget University Hospital, Gorleston-on-Sea, United Kingdom. Abstract Introduction: Phacomatosis pigmentovascularis is a term encompassing a group of disorders characterized by the coexistence of a segmental pigmented nevus of melanocytic origin and segmental capillary nevus. Over the past decades, confusion over the names and definitions of phacomatosis spilorosea, phacomatosis melanorosea, and their defining nevi, as well as of unclassifi- able phacomatosis pigmentovascularis cases, has led to several misplaced diagnoses in published cases. Methods: A systematic and critical review of the worldwide literature on phacomatosis spilorosea and phacomatosis melanorosea was carried out. Results: This study yielded 18 definite instances of phacomatosis spilorosea and 14 of phacomatosis melanorosea, with one and six previously unrecognized cases, respectively. Conclusions: Phacomatosis spilorosea predominantly involves the musculoskeletal system and can be complicated by neuro- logical manifestations. Phacomatosis melanorosea is sometimes associated with ancillary cutaneous lesions, displays a relevant association with vascular malformations of the brain, and in general appears to be a less severe syndrome. -

Oral Pathology
Oral Pathology Palatal blue nevus in a child Catherine M. Flaitz DDS, MS Georgeanne McCandless DDS Dr. Flaitz is professor, Oral and Maxillofacial Pathology and Pediatric Dentistry, Department of Stomatology, University of Texas at Houston Health Science Center Dental Branch; Dr. McCandless has a private practice in The Woodlands, TX. Correspond with Dr. Flaitz at [email protected] Abstract The intraoral blue nevus occurs infrequently in children. This by the labial mucosa (1). Intraoral lesions have a predilection case report describes the clinical features of an acquired blue ne- for females in the third and fourth decades, in contrast to cu- vus in a 7 year-old girl that involved the palatal mucosa. A taneous lesions that normally develop in children. In large differential diagnosis and justification for surgical excision of this biopsy series, only 2% of the oral blue nevi are diagnosed in oral lesion are discussed. (Pediatr Dent 23:354-355, 2001) children and adolescents (1). Similar to their cutaneous coun- terpart, most oral lesions are acquired; however, there are ith the exception of vascular entities, neoplastic isolated reports of congenital examples. lesions with a blue discoloration are an unusual find Clinically, most lesions present as a solitary blue, gray or Wing in children. Although the blue nevus is a blue-black macule or slightly raised nodule that measures less relatively common finding of the skin in the pediatric popula- than 6 mm in size. The margins are often regular but indis- tion, only a few intraoral examples are documented in the tinct and the surface is smooth. -

Optimal Management of Common Acquired Melanocytic Nevi (Moles): Current Perspectives
Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Optimal management of common acquired melanocytic nevi (moles): current perspectives Kabir Sardana Abstract: Although common acquired melanocytic nevi are largely benign, they are probably Payal Chakravarty one of the most common indications for cosmetic surgery encountered by dermatologists. With Khushbu Goel recent advances, noninvasive tools can largely determine the potential for malignancy, although they cannot supplant histology. Although surgical shave excision with its myriad modifications Department of Dermatology and STD, Maulana Azad Medical College and has been in vogue for decades, the lack of an adequate histological sample, the largely blind Lok Nayak Hospital, New Delhi, Delhi, nature of the procedure, and the possibility of recurrence are persisting issues. Pigment-specific India lasers were initially used in the Q-switched mode, which was based on the thermal relaxation time of the melanocyte (size 7 µm; 1 µsec), which is not the primary target in melanocytic nevus. The cluster of nevus cells (100 µm) probably lends itself to treatment with a millisecond laser rather than a nanosecond laser. Thus, normal mode pigment-specific lasers and pulsed ablative lasers (CO2/erbium [Er]:yttrium aluminum garnet [YAG]) are more suited to treat acquired melanocytic nevi. The complexities of treating this disorder can be overcome by following a structured approach by using lasers that achieve the appropriate depth to treat the three subtypes of nevi: junctional, compound, and dermal. Thus, junctional nevi respond to Q-switched/normal mode pigment lasers, where for the compound and dermal nevi, pulsed ablative laser (CO2/ Er:YAG) may be needed. -

Hutchinson's Melanotic Freckle
Hutchiso’s elaotic freckle Also known as Lentigo maligna What is lentigo maligna? Lentigo maligna is an early form of melanoma. In lentigo maligna the cancer cells are confined to the upper layer of the skin (epidermis). When the cancer cells spread deeper into the skin (to dermis) it is called lentigo maligna melanoma. Lentigo maligna occurs most commonly in sun damaged areas such as the face and neck in fair skinned people over the age of 60. The lesion grows slowly in size over a number of years. Melanoma is a potentially lethal disease and lentigo maligna should be diagnosed and excised as soon as possible. What causes lentigo maligna? The cause of lentigo maligna is sun exposure or solarium use. Factors that predispose a person to developing lentigo maligna or associated condition include: . chronic sun damaged/solar-induced skin damage . fair skin complexion . male gender . a personal history of non melanoma skin cancer and precancerous lesions . older individuals (those between 60 to 80 years are most commonly affected) What does lentigo maligna look like? Lentigo maligna commonly looks like a freckle, age spot, sun spot or brown patch that slowly changes shape and grows in size. The spot may be large in size, irregularly shaped with a smooth surface, and of multiple shades of brown and sometimes other colours. Thickening of part of the lesion, increasing number of colours, ulceration or bleeding can be markers that the lesion is changing into a lentigo maligna melanoma. How is lentigo maligna diagnosed? Lentigo maligna is diagnosed clinically by a dermatologist, sometimes with the help of a dermatoscope (a tool used to magnify and look closely at skin moles). -

Identification of HRAS Mutations and Absence of GNAQ Or GNA11
Modern Pathology (2013) 26, 1320–1328 1320 & 2013 USCAP, Inc All rights reserved 0893-3952/13 $32.00 Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi Ryan P Bender1, Matthew J McGinniss2, Paula Esmay1, Elsa F Velazquez3,4 and Julie DR Reimann3,4 1Caris Life Sciences, Phoenix, AZ, USA; 2Genoptix Medical Laboratory, Carlsbad, CA, USA; 3Dermatopathology Division, Miraca Life Sciences Research Institute, Newton, MA, USA and 4Department of Dermatology, Tufts Medical Center, Boston, MA, USA HRAS is mutated in B15% of Spitz nevi, and GNAQ or GNA11 is mutated in blue nevi (46–83% and B7% respectively). Epithelioid blue nevi and deep penetrating nevi show features of both blue nevi (intradermal location, pigmentation) and Spitz nevi (epithelioid morphology). Epithelioid blue nevi and deep penetrating nevi can also show overlapping features with melanoma, posing a diagnostic challenge. Although epithelioid blue nevi are considered blue nevic variants, no GNAQ or GNA11 mutations have been reported. Classification of deep penetrating nevi as blue nevic variants has also been proposed, however, no GNAQ or GNA11 mutations have been reported and none have been tested for HRAS mutations. To better characterize these tumors, we performed mutational analysis for GNAQ, GNA11, and HRAS, with blue nevi and Spitz nevi as controls. Within deep penetrating nevi, none demonstrated GNAQ or GNA11 mutations (0/38). However, 6% revealed HRAS mutation (2/32). Twenty percent of epithelioid blue nevi contained a GNAQ mutation (2/10), while none displayed GNA11 or HRAS mutation. Eighty-seven percent of blue nevi contained a GNAQ mutation (26/30), 4% a GNA11 mutation (1/28), and none an HRAS mutation. -

Pigmented Contact Dermatitis and Chemical Depigmentation
18_319_334* 05.11.2005 10:30 Uhr Seite 319 Chapter 18 Pigmented Contact Dermatitis 18 and Chemical Depigmentation Hideo Nakayama Contents ca, often occurs without showing any positive mani- 18.1 Hyperpigmentation Associated festations of dermatitis such as marked erythema, with Contact Dermatitis . 319 vesiculation, swelling, papules, rough skin or scaling. 18.1.1 Classification . 319 Therefore, patients may complain only of a pigmen- 18.1.2 Pigmented Contact Dermatitis . 320 tary disorder, even though the disease is entirely the 18.1.2.1 History and Causative Agents . 320 result of allergic contact dermatitis. Hyperpigmenta- 18.1.2.2 Differential Diagnosis . 323 tion caused by incontinentia pigmenti histologica 18.1.2.3 Prevention and Treatment . 323 has often been called a lichenoid reaction, since the 18.1.3 Pigmented Cosmetic Dermatitis . 324 presence of basal liquefaction degeneration, the ac- 18.1.3.1 Signs . 324 cumulation of melanin pigment, and the mononucle- 18.1.3.2 Causative Allergens . 325 ar cell infiltrate in the upper dermis are very similar 18.1.3.3 Treatment . 326 to the histopathological manifestations of lichen pla- 18.1.4 Purpuric Dermatitis . 328 nus. However, compared with typical lichen planus, 18.1.5 “Dirty Neck” of Atopic Eczema . 329 hyperkeratosis is usually milder, hypergranulosis 18.2 Depigmentation from Contact and saw-tooth-shape acanthosis are lacking, hyaline with Chemicals . 330 bodies are hardly seen, and the band-like massive in- 18.2.1 Mechanism of Leukoderma filtration with lymphocytes and histiocytes is lack- due to Chemicals . 330 ing. 18.2.2 Contact Leukoderma Caused Mainly by Contact Sensitization .