Search Strategies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidance on Identification of Alternatives to New Pops
Stockholm Convention on Persistent Organic Pollutants Guidance on identification of alternatives to new POPs Secretariat of the Stockholm Convention Concept of “Substitution” under the Stockholm Convention • The substitution is a strategy promoted by the Stockholm Convention to reach its objectives • Parties that are still producing or using the new POPs listed in Annex A, will need to search and identify alternatives to replace them • In the case of PFOS and for the exemptions for uses allowed by the Convention, these group of chemicals will be eventually prohibited and Parties are therefore encouraged to find alternatives to substitute them 2 Availability of alternatives • Currently, some countries have phased out the use of some of the new POPs, and there are feasible alternatives available to replace them Alternatives Chemical Name Use Ethoprop, oxamyl Pesticide to control banana root borer Cyfluthrin, Imidacloprid Pesticide to control tobacco wireworms Azadirachtin, bifenthrin, boric acid, carbaryl, Pesticide to control capsaicin, cypermethrin, cyfluthrin, ants and/or deltamethrin, diazinon, dichlorvos, cockroaches esfenvalerate, imidacloprid, lamda-cyhalothrin, Chlordecone malathion, permethrin, piperonyl butoxide, pyrethrins, pyriproxyfen, resmethrin, s- bioallerthrin, tetramethrin Bacillus thuringiensis, cultural practices such Pest management as crop rotation, intercropping, and trap cropping; barrier methods, such as screens, and bagging of fruit; use of traps such as pheromone and light traps to attract and kill insects. 3 -

Federal Register/Vol. 75, No. 146/Friday, July 30, 2010/Notices
44954 Federal Register / Vol. 75, No. 146 / Friday, July 30, 2010 / Notices 4. Submission of Your Response in the of the information? If so, please attach must be submitted for inclusion in the English Language a copy of the determination. public docket. All responses to this notice must be 6. For each category of information 2. Tips for Preparing Your Comments. in the English language. claimed as confidential, explain with When submitting comments, remember specificity why release of the to: 5. The Effect of Failure To Respond to information is likely to cause substantial • Identify the notice by docket This Notice harm to your competitive position. number and other identifying In accordance with 40 CFR 2.204(e)(1) Explain the specific nature of those information (subject heading, Federal harmful effects, why they should be Register date and page number). and 2.205(d)(1), EPA will construe your • failure to furnish timely comments in viewed as substantial, and the causal Explain your views as clearly as response to this notice as a waiver of relationship between disclosure and possible, avoiding the use of profanity such harmful effects. How could your or personal threats. your business’s claim(s) of • confidentiality for any information in competitors make use of this Describe any assumptions and the types of documents identified in this information to your detriment? provide any technical information and/ notice. 7. Do you assert that the information or data that you used. is submitted on a voluntary or a • Provide specific examples to 6. What To Include in Your Comments mandatory basis? Please explain the illustrate your concerns, and suggest If you believe that any of the reason for your assertion. -

Federal Register/Vol. 75, No. 115/Wednesday, June 16, 2010
34126 Federal Register / Vol. 75, No. 115 / Wednesday, June 16, 2010 / Notices TABLE 2.—REGISTRANTS REQUESTING TABLE 2.—REGISTRANTS REQUESTING the cancellation action. Because the VOLUNTARY CANCELLATION—Con- VOLUNTARY CANCELLATION—Con- Agency has identified no significant tinued tinued potential risk concerns associated with these pesticide products, upon cancellation of the products identified Company Name and EPA Co. Number Company Name and EPA Co. Number Address Address in Table 1 of Unit II., EPA anticipates allowing registrants to sell and AZ970004; Chemtura Corpora- MN940003 Arysta Lifescience distribute existing stocks of these OR030022; tion North America, products for 1 year after publication of LLC WA910017 ATTN: Crop Reg- the Cancellation Order in the Federal istration, Michael 155401 Weston Dupre Parkway, Suite Register. Thereafter, registrants will be 199 Benson Road 150 prohibited from selling or distributing (2-5) Cary, NC 27513 the pesticides identified in Table 1 of Middlebury, CT Unit II., except for export consistent 06749 III. What is the Agency’s Authority for with FIFRA section 17 or for proper Taking this Action? disposal. Persons other than registrants OR910006; FMC Corp., Agricul- will generally be allowed to sell, Section 6(f)(1) of FIFRA provides that CO920001 tural Products distribute, or use existing stocks until a registrant of a pesticide product may Group such stocks are exhausted, provided that ATTN: Michael C. at any time request that any of its such sale, distribution, or use is Zucker pesticide registrations be canceled. consistent with the terms of the 1735 Market St., FIFRA further provides that, before previously approved labeling on, or that RM. -

Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom
Clinical Evidence Handbook A Publication of BMJ Publishing Group Scabies PAUL JOHNSTONE, Blenheim House, Leeds, United Kingdom MARK STRONG, University of Sheffield, Sheffield, United Kingdom This is one in a series of Scabies is an infestation of the skin by the • Although tested in randomized chapters excerpted from mite Sarcoptes scabiei. In adults, the most controlled trials (RCTs), oral ivermectin is the Clinical Evidence Handbook, published by common sites of infestation are the fingers not presently licensed for the treatment of the BMJ Publishing Group, and the wrists, although it may manifest in scabies in most countries. It is only avail- London, U.K. The medical older persons as a diffuse truncal eruption. able on a named patient basis in the United information contained herein is the most accurate • Scabies is a very common public health Kingdom. available at the date of problem. In many resource-poor settings, it Topical lindane use has been restricted publication. More updated is an endemic problem; whereas in indus- or is not available in many parts of the and comprehensive infor- trialized countries, it is most common in world owing to the mounting evidence mation on this topic may be available in future print institutionalized communities. for serious adverse effects. We have not editions of the Clinical Evi- • Topical permethrin seems highly effec- included it in this review. However, it may dence Handbook, as well tive at increasing clinical cure of scabies be the most effective treatment that is as online at http://www. within 28 days. locally available in some countries. Harms clinicalevidence.bmj.com (subscription required). -

TITLE: Lindane and Other Treatments for Lice and Scabies: a Review of Clinical Effectiveness and Safety
TITLE: Lindane and Other Treatments for Lice and Scabies: A Review of Clinical Effectiveness and Safety DATE: 11 June 2010 CONTEXT AND POLICY ISSUES: Head lice infestation (Pediculosis capitis) affects millions of children and adults worldwide each year.1 Direct head-to-head contact is the most common mode of transmission.2 The highest prevalence of infestation occurs in school aged children aged three to eleven years, with girls being more commonly affected than boys.1,2 Although head lice are not generally associated with serious morbidity, they are responsible for significant social embarrassment and lost productivity in schools or offices.1 Scabies, an infestation of the skin by the mite Sarcoptes scabiei, represents a common public health concern particularly in overcrowded communities with a high prevalence of poverty.3 Scabies is transmitted by close-person contact and occasionally by clothing or linens.3 Complications include secondary bacterial infections and post-streptococcal glomerulonephritis.3 Topical products available in Canada for the treatment of head lice and scabies are presented in Appendix 1 and Appendix 2. Insecticidal agents such as permethrin and lindane have historically been considered the standard treatments for head lice and scabies.2,3 Toxicity is low following topical administration of permethrin due to minimal percutaneous absorption.4 However, several jurisdictions have banned lindane due to concerns of neurotoxicity and bone marrow suppression, as well as potential negative effects on the environment (contamination of waste water).5 Furthermore, widespread use of permethrin, pyrethrins/piperonyl butoxide, and lindane has led to resistance and higher rates of treatment failure.6 Resistance patterns and rates to these agents in Canada have not yet been studied.6 Due to concerns surrounding resistance and neurotoxicity, patients and caregivers have searched for alternative treatments. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 5/10/2013 Revision Date: 10/2/2018 Revision Number: G4 1. IDENTIFICATION Product identifier Product code: BE159 Product Name: BENZYL BENZOATE, USP Other means of identification Synonyms: Ascabin Ascabiol Benylate Benzyl alcohol benzoic ester Benzyl benzenecarboxylate Benzylets Benzyl phenylformate Benzoic acid, phenylmethyl ester Colebenz Novoscabin Peruscabin Scabanca Vanzoate Venzonate CAS #: 120-51-4 RTECS # DG4200000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Plasticizer for cellulose. Fixative. Dye carrier. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Oral Category 4 Product code: BE159 Product name: BENZYL BENZOATE, 1 / 12 USP Label elements Warning Hazard statements Harmful if swallowed Hazards not otherwise classified (HNOC) Not Applicable Other hazards Toxic to aquatic life with long lasting effects May be harmful in contact with skin Precautionary Statements - Prevention Wash face, hands and any exposed skin thoroughly after handling Do not eat, drink or smoke when using this product Precautionary Statements - Response IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell Rinse mouth Precautionary Statements - Disposal Dispose of contents/container to an approved waste disposal plant 3. -

Nebido 1000 Mg/4 Ml, Solution for Injection Testosterone Undecanoate
Bayer plc 400 South Oak Way, Reading, RG2 6AD Telephone: +44 (0) 118 206 3000 Medical information: [email protected]. www: http://www.bayer.co.uk Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end of the leaflet to establish if there have been any changes. If you have any doubts or queries about your medication, please contact your doctor or pharmacist. Package leaflet: Information for the user Nebido 1000 mg/4 ml, solution for injection Testosterone undecanoate Read all of this leaflet carefully before you are given this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Nebido is and what it is used for 2. What you need to know before you are given Nebido 3. How to use Nebido 4. Possible side effects 5. How to store Nebido 6. Contents of the pack and other information 1. What Nebido is and what it is used for Nebido contains testosterone, a male hormone, as the active ingredient. -

UNEP-POPS-POPRC.3-20-Add.4
UNITED NATIONS SC UNEP/POPS/POPRC.3/20/Add.4 Distr.: General 4 December 2007 United Nations English only Environment Programme Stockholm Convention on Persistent Organic Pollutants Persistent Organic Pollutants Review Committee Third meeting Geneva, 19–23 November 2007 Report of the Persistent Organic Pollutants Review Committee on the work of its third meeting Addendum Risk management evaluation on lindane At its third meeting, the Persistent Organic Pollutants Review Committee adopted the risk management evaluation on lindane, on the basis of the draft contained in document UNEP/POPS/POPRC.3/12. The text of the risk management evaluation, as amended, is set out below. It has not been formally edited. K0763732 181207 UNEP/POPS/POPRC.3/20/Add.4 LINDANE RISK MANAGEMENT EVALUATION Adopted by the Persistent Organic Pollutants Review Committee at its third meeting November 2007 2 UNEP/POPS/POPRC.3/20/Add.4 TABLE OF CONTENTS Executive summary .................................................................................................................................................................. 4 1. Introduction..................................................................................................................................................................... 5 1.1 Chemical identity of the proposed substance ........................................................................................................5 1.2 Conclusions of the Review Committee .................................................................................................................6 -
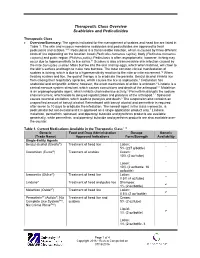
Therapeutic Class Overview Scabicides and Pediculicides
Therapeutic Class Overview Scabicides and Pediculicides Therapeutic Class Overview/Summary: The agents indicated for the management of scabies and head lice are listed in Table 1. The skin and mucous membrane scabicides and pediculicides are approved to treat pediculosis and scabies.1-10 Pediculosis is a transmissible infection, which is caused by three different kinds of lice depending on the location: head (Pediculus humanus capitis), body (Pediculus humanus corporis) and pubic region (Phthirus pubis). Pediculosis is often asymptomatic; however, itching may occur due to hypersensitivity to lice saliva.11 Scabies is also a transmissible skin infection caused by the mite Sarcoptes scabiei. Mites burrow into the skin and lay eggs, which when hatched, will crawl to the skin’s surface and begin to make new burrows. The most common clinical manifestation of scabies is itching, which is due to a hypersensitivity reaction to the mite or mite excrement.12 When treating scabies and lice, the goal of therapy is to eradicate the parasite. Benzyl alcohol inhibits lice from closing their respiratory spiracles, which causes the lice to asphyxiate.3 Crotamiton has scabicidal and antipruritic actions; however, the exact mechanism of action is unknown.4 Lindane is a central nervous system stimulant, which causes convulsions and death of the arthropod.1,2 Malathion is an organophosphate agent, which inhibits cholinesterase activity.5 Permethrin disrupts the sodium channel current, which leads to delayed repolarization and paralysis of the arthropod.1,2 Spinosad causes neuronal excitation, which leads to paralysis and death.6 The suspension also contains an unspecified amount of benzyl alcohol. -

Environmental Health Criteria 87 Allethrins
Environmental Health Criteria 87 Allethrins Please note that the layout and pagination of this web version are not identical with the printed version. Allethrins (EHC 87, 1989) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 87 ALLETHRINS - Allethrin - d-Allethrin - Bioallethrin - S-Bioallethrin This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1989 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. ISBN 92 4 154287 X The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations Page 1 of 46 Allethrins (EHC 87, 1989) already available. -

Injection Read This Medication Guide Before You Receive AVEED and Before Each Injection
MEDICATION GUIDE AVEED® (Uh-Veed) (testosterone undecanoate) injection Read this Medication Guide before you receive AVEED and before each injection. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. What is the most important information I should know about AVEED? AVEED may cause serious side effects, including: • A serious lung problem. AVEED can cause a serious lung problem called a pulmonary oil microembolism (POME) reaction. POME is caused by tiny droplets of oil that have traveled to the lungs. Symptoms of a POME reaction may include: − cough or urge to cough − difficulty breathing − sweating − tightening of your throat − chest pain − dizziness − fainting • Serious allergic reactions (anaphylaxis). AVEED can cause a serious allergic reaction right after receiving the injection. Some of these allergic reactions may be life threatening. These reactions can happen after you receive your first dose of AVEED or may happen after receiving more than 1 dose. You may need emergency treatment in a hospital, especially if these symptoms get worse over the 24 hours after your AVEED injection. These side effects may happen during or right after each injection. To be sure that you are not having one of these reactions: • You need to stay in the doctor’s office, clinic, or hospital for 30 minutes after having your AVEED injection so that your doctor can watch you for symptoms of POME or a serious allergic reaction. • You can only get AVEED at your doctor’s office, clinic, or hospital. AVEED is only available through a restricted program called the AVEED Risk Evaluation and Mitigation Strategy (REMS) Program. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01