The National Plant Biosecurity Status Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Addenda and Amendments to a Checklist of the Lepidoptera of the British Isles on Account of Subsequently Published Data
Ent Rec 128(2)_Layout 1 22/03/2016 12:53 Page 98 94 Entomologist’s Rec. J. Var. 128 (2016) ADDENDA AND AMENDMENTS TO A CHECKLIST OF THE LEPIDOPTERA OF THE BRITISH ISLES ON ACCOUNT OF SUBSEQUENTLY PUBLISHED DATA 1 DAVID J. L. A GASSIZ , 2 S. D. B EAVAN & 1 R. J. H ECKFORD 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD 2 The Hayes, Zeal Monachorum, Devon EX17 6DF This update incorpotes information published before 25 March 2016 into A Checklist of the Lepidoptera of the British Isles, 2013. CENSUS The number of species now recorded from the British Isles stands at 2535 of which 57 are thought to be extinct and in addition there are 177 adventive species. CHANGE OF STATUS (no longer extinct) p. 17 16.013 remove X, Hall (2013) p. 25 35.006 remove X, Beavan & Heckford (2014) p. 40 45.024 remove X, Wilton (2014) p. 54 49.340 remove X, Manning (2015) ADDITIONAL SPECIES in main list 12.0047 Infurcitinea teriolella (Amsel, 1954) E S W I C 15.0321 Parornix atripalpella Wahlström, 1979 E S W I C 15.0861 Phyllonorycter apparella (Herrich-Schäffer, 1855) E S W I C 15.0862 Phyllonorycter pastorella (Zeller, 1846) E S W I C 27.0021 Oegoconia novimundi (Busck, 1915) E S W I C 35.0299 Helcystogramma triannulella (Herrich-Sch äffer, 1854) E S W I C 41.0041 Blastobasis maroccanella Amsel, 1952 E S W I C 48.0071 Choreutis nemorana (Hübner, 1799) E S W I C 49.0371 Clepsis dumicolana (Zeller, 1847) E S W I C 49.2001 TETRAMOERA Diakonoff, [1968] langmaidi Plant, 2014 E S W I C 62.0151 Delplanqueia inscriptella (Duponchel, 1836) E S W I C 72.0061 Hypena lividalis (Hübner, 1790) Chevron Snout E S W I C 70.2841 PUNGELARIA Rougemont, 1903 capreolaria ([Denis & Schiffermüller], 1775) Banded Pine Carpet E S W I C 72.0211 HYPHANTRIA Harris, 1841 cunea (Drury, 1773) Autumn Webworm E S W I C 73.0041 Thysanoplusia daubei (Boisduval, 1840) Boathouse Gem E S W I C 73.0301 Aedia funesta (Esper, 1786) Druid E S W I C Ent Rec 128(2)_Layout 1 22/03/2016 12:53 Page 99 Entomologist’s Rec. -

The Microlepidopterous Fauna of Sri Lanka, Formerly Ceylon, Is Famous
ON A COLLECTION OF SOME FAMILIES OF MICRO- LEPIDOPTERA FROM SRI LANKA (CEYLON) by A. DIAKONOFF Rijksmuseum van Natuurlijke Historie, Leiden With 65 text-figures and 18 plates CONTENTS Preface 3 Cochylidae 5 Tortricidae, Olethreutinae, Grapholitini 8 „ „ Eucosmini 23 „ „ Olethreutini 66 „ Chlidanotinae, Chlidanotini 78 „ „ Polyorthini 79 „ „ Hilarographini 81 „ „ Phricanthini 81 „ Tortricinae, Tortricini 83 „ „ Archipini 95 Brachodidae 98 Choreutidae 102 Carposinidae 103 Glyphipterigidae 108 A list of identified species no A list of collecting localities 114 Index of insect names 117 Index of latin plant names 122 PREFACE The microlepidopterous fauna of Sri Lanka, formerly Ceylon, is famous for its richness and variety, due, without doubt, to the diversified biotopes and landscapes of this beautiful island. In spite of this, there does not exist a survey of its fauna — except a single contribution, by Lord Walsingham, in Moore's "Lepidoptera of Ceylon", already almost a hundred years old, and a number of small papers and stray descriptions of new species, in various journals. The authors of these papers were Walker, Zeller, Lord Walsingham and a few other classics — until, starting with 1905, a flood of new descriptions 4 ZOOLOGISCHE VERHANDELINGEN I93 (1982) and records from India and Ceylon appeared, all by the hand of Edward Meyrick. He was almost the single specialist of these faunas, until his death in 1938. To this great Lepidopterist we chiefly owe our knowledge of all groups of Microlepidoptera of Sri Lanka. After his death this information stopped abruptly. In the later years great changes have taken place in the tropical countries. We are now facing, alas, the disastrously quick destruction of natural bio- topes, especially by the reckless liquidation of the tropical forests. -

Description of Sexuales of Brachycolus Cucubali (Passerini, 1863) (Hemiptera Aphididae)
REDIA, 103, 2020: 47-53 http://dx.doi.org/10.19263/REDIA-103.20.09 ALICE CASIRAGHIa,b -VÍCTOR MORENO-GONZÁLEZ c - NICOLÁS PÉREZ HIDALGO a, d DESCRIPTION OF SEXUALES OF BRACHYCOLUS CUCUBALI (PASSERINI, 1863) (HEMIPTERA APHIDIDAE) a) Instituto de Biología Integrativa de Sistemas (I2SysBio). Centro Mixto Universidad de Valencia-CSIC (Paterna, Va- lencia). Spain. b) Agroecosystems Research Group, Biodiversity Research Institute (IRBio), Section of Botany and Mycology, Depart- ment of Evolutionary Biology, Ecology and Environmental Sciences, Faculty of Biology, University of Barcelona, Avda. Diagonal 643, Barcelona, Spain. ORCID ID: https://orcid.org/0000-0003-0955-8353 c) Departamento de Biodiversidad y Gestión Ambiental, área de Zoología. Universidad de León. 24071 León Spain. ORCID ID: https://orcid.org/0000-0003-0094-1559 d) Departamento de Artrópodos, Museo de Ciencias Naturales de Barcelona, 08003, Barcelona, Spain. ORCID ID: http://orcid.org/0000-0001-8143-3366 Corresponding Author: Alice Casiraghi; [email protected] Casiraghi A., Moreno-González V., Pérez Hidalgo, N. – Description of sexuales of Brachycolus cucubali (Passerini 1863) [Hemiptera Aphididae]. The hitherto unknown oviparous females and apterous males of Brachycolus cucubali (Passerini, 1863), living in pseudogalls on Silene vulgaris (Moench) Garcke, (1869) (Caryophyllaceae), are described based on material from the North-West of Iberian Peninsula (Province of León). Sampling and morphometric data are given for every morph. Also, field data of monitored Brachycolus cucubali colonies are reported and information of polyphenism in males is discussed. KEY WORDS: Aphids, morphology, sexual dimorphism, polyphenism INTRODUCTION 2014), or with the species of this work, Brachycolus cucu- bali (Passerini, 1863) (BLACKMAN and EASTOP, 2020). -

A Contribution to the Aphid Fauna of Greece
Bulletin of Insectology 60 (1): 31-38, 2007 ISSN 1721-8861 A contribution to the aphid fauna of Greece 1,5 2 1,6 3 John A. TSITSIPIS , Nikos I. KATIS , John T. MARGARITOPOULOS , Dionyssios P. LYKOURESSIS , 4 1,7 1 3 Apostolos D. AVGELIS , Ioanna GARGALIANOU , Kostas D. ZARPAS , Dionyssios Ch. PERDIKIS , 2 Aristides PAPAPANAYOTOU 1Laboratory of Entomology and Agricultural Zoology, Department of Agriculture Crop Production and Rural Environment, University of Thessaly, Nea Ionia, Magnesia, Greece 2Laboratory of Plant Pathology, Department of Agriculture, Aristotle University of Thessaloniki, Greece 3Laboratory of Agricultural Zoology and Entomology, Agricultural University of Athens, Greece 4Plant Virology Laboratory, Plant Protection Institute of Heraklion, National Agricultural Research Foundation (N.AG.RE.F.), Heraklion, Crete, Greece 5Present address: Amfikleia, Fthiotida, Greece 6Present address: Institute of Technology and Management of Agricultural Ecosystems, Center for Research and Technology, Technology Park of Thessaly, Volos, Magnesia, Greece 7Present address: Department of Biology-Biotechnology, University of Thessaly, Larissa, Greece Abstract In the present study a list of the aphid species recorded in Greece is provided. The list includes records before 1992, which have been published in previous papers, as well as data from an almost ten-year survey using Rothamsted suction traps and Moericke traps. The recorded aphidofauna consisted of 301 species. The family Aphididae is represented by 13 subfamilies and 120 genera (300 species), while only one genus (1 species) belongs to Phylloxeridae. The aphid fauna is dominated by the subfamily Aphidi- nae (57.1 and 68.4 % of the total number of genera and species, respectively), especially the tribe Macrosiphini, and to a lesser extent the subfamily Eriosomatinae (12.6 and 8.3 % of the total number of genera and species, respectively). -

Downloaded from BOLD Or Requested from Other Authors
www.nature.com/scientificreports OPEN Towards a global DNA barcode reference library for quarantine identifcations of lepidopteran Received: 28 November 2018 Accepted: 5 April 2019 stemborers, with an emphasis on Published: xx xx xxxx sugarcane pests Timothy R. C. Lee 1, Stacey J. Anderson2, Lucy T. T. Tran-Nguyen3, Nader Sallam4, Bruno P. Le Ru5,6, Desmond Conlong7,8, Kevin Powell 9, Andrew Ward10 & Andrew Mitchell1 Lepidopteran stemborers are among the most damaging agricultural pests worldwide, able to reduce crop yields by up to 40%. Sugarcane is the world’s most prolifc crop, and several stemborer species from the families Noctuidae, Tortricidae, Crambidae and Pyralidae attack sugarcane. Australia is currently free of the most damaging stemborers, but biosecurity eforts are hampered by the difculty in morphologically distinguishing stemborer species. Here we assess the utility of DNA barcoding in identifying stemborer pest species. We review the current state of the COI barcode sequence library for sugarcane stemborers, assembling a dataset of 1297 sequences from 64 species. Sequences were from specimens collected and identifed in this study, downloaded from BOLD or requested from other authors. We performed species delimitation analyses to assess species diversity and the efectiveness of barcoding in this group. Seven species exhibited <0.03 K2P interspecifc diversity, indicating that diagnostic barcoding will work well in most of the studied taxa. We identifed 24 instances of identifcation errors in the online database, which has hampered unambiguous stemborer identifcation using barcodes. Instances of very high within-species diversity indicate that nuclear markers (e.g. 18S, 28S) and additional morphological data (genitalia dissection of all lineages) are needed to confrm species boundaries. -

Pest Insects Infesting Carrot and Other Apiaceous Crops
FACTSHEET 01/16 Field Vegetables Dr Rosemary Collier, University of Warwick Pest insects infesting carrot and other Apiaceous crops Carrot and related Apiaceous crops such as parsnip, celeriac and celery may be infested by a relatively small number of damaging pest insect species that can reduce both quality and yield. This factsheet focuses on the pest insects infesting carrot. However, the insects described may also be pests of related crops such as parsnip, celeriac and celery. The factsheet does not cover nematodes or slugs. Figure 1. Carrot fly damage to carrot roots Action points Carrot fly • Use suction trap records to indicate when all species of pest aphid are on the wing. • Use the carrot fly forecast to indicate when adult flies of each generation are likely to emerge and when the • Monitor crops closely. application of insecticide sprays is likely to be most effective. • Use the most effective aphicide treatments available, • Check whether an insecticide spray treatment is able taking account of their effects on natural enemies and be to kill adults or larvae or both stages. To maximise the aware of potential instances of insecticide resistance. ‘knockdown’ effect of insecticide sprays that target adults, they should be applied between 4–6pm on warm days, as Cutworms this is when most female flies are in the crop. • Use pheromone traps to indicate when turnip moths are • Where possible, isolate new crops from possible sources flying and laying eggs. of infestation (older crops), ideally using a separation distance of >1km. • Use a cutworm forecast to estimate the risk of damage to susceptible crops. -

25 Host Plant Resistance and Insect Pest Management in Chickpea
25 Host Plant Resistance and Insect Pest Management in Chickpea H.C. SHARMA,1 C.L.L. GOWDA,1 P.C. STEVENSON,2 T.J. RIDSDILL-SMITH,3 S.L. CLEMENT,4 G.V. RANGA RAO,1 J. ROMEIS,5 M. MILES6 AND M. EL BOUHSSINI7 1Genetics Resources Divisions, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Asia Center, Hyderabad 502324, India; 2Natural Resources Institute, University of Greenwich, Chatham, ME4 4TB, UK and Royal Botanic Gardens, Kew, Surrey, TW9 3AB, UK; 3CSIRO Entomology, Private Bag No 5, Wembley, WA 6913, Australia; 4USDA-ARS, 59 Johnson Hall, Washington State University, Pullman, WA 99164-6402, USA; 5Agroscope ART Reckenholz Tänikon, Reckenholzstr, 191, 8046 Zurich, Switzerland; 6Department of Primary Industries and Fisheries, 203 Tor Street, PO Box 102, Toowoomba, Queensland 4350, Australia; 7International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria Insect Pest Problems in Chickpea Chickpea (C. arietinum L.) is the third most important legume crop in the world, after dry beans and peas (FAO, 2003). It is cultivated in 42 countries in South Asia, North and Central America, the Mediterranean region, West Asia and North and East Africa. In recent years, it has become an important crop in Australia, Canada and the USA. Nearly 60 insect species are known to feed on chickpea (Reed et al., 1987) (Table 25.1). The important insect pests damaging chickpea in different regions are: ● Wireworms: false wireworm – Gonocephalum spp.; ● Cutworm: black cutworm – A. ipsilon (Hfn.) and turnip moth – A. segetum Schiff.; ● Termite: Microtermes obesi (Holm.) and Odontotermes sp.; ● Leaf-feeding caterpillars: cabbage looper – Trichoplusia ni (Hub.), leaf caterpillar – S. -

Biodiversity – Economy Or Ecology? Long-Term Study of Changes in the Biodiversity of Aphids Living in Steppe-Like Grasslands in Central Europe
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 114: 140–146, 2017 http://www.eje.cz doi: 10.14411/eje.2017.019 ORIGINAL ARTICLE Biodiversity – economy or ecology? Long-term study of changes in the biodiversity of aphids living in steppe-like grasslands in Central Europe BARBARA OSIADACZ 1, ROMAN HAŁAJ 2 and DAMIAN CHMURA3 1 Department of Entomology and Environmental Protection, Poznań University of Life Sciences, Dąbrowskiego St. 159, PL 60-594 Poznań, Poland; e-mail: [email protected] 2 The Upper Silesian Nature Society, Huberta St. 35, PL 40-543 Katowice, Poland; e-mail: [email protected] 3 Institute of Environmental Protection and Engineering, University of Bielsko-Biała, Willowa 2, PL 43-309 Bielsko-Biała, Poland; e-mail: [email protected] Key words. Hemiptera, Aphidoidea, bio-ecological groups, community structure, protected habitats, loss of biodiversity, human impact, NMDS methods, regional hotspots Abstract. This paper examines the changes in the species composition of aphids living in dry calcareous grasslands in Central Europe over a 25-year period. To the best of our knowledge, this is the fi rst analysis of this type in the world that takes into account both previous and current data on species richness as well as groups of aphids that are distinguishable on the basis of biological and ecological criteria such as host-alternation and feeding types, life cycle, ecological niche, symbiosis with ants and their eco- logical functional groups. Over the period of more than 25 years, there has been a signifi cant decrease in aphid α-diversity, from 171 to 105 species. -
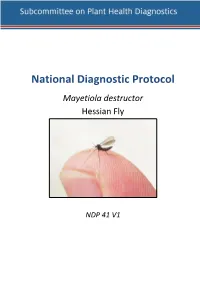
NDP 41 Hessian
NDP 41 V1- National Diagnostic Protocol for Mayetiola destructor National Diagnostic Protocol Mayetiola destructor Hessian Fly NDP 41 V1 NDP 41 V1 - National Diagnostic Protocol for Mayetiola destructor © Commonwealth of Australia Ownership of intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth). Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from http://creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from https://creativecommons.org/licenses/by/3.0/au/legalcode. This publication (and any material sourced from it) should be attributed as: Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee on Plant Health Diagnostics) Authors Severtson, D, Szito, A.; Reviewers Nicholas, A, Kehoe, M. ISBN 978-0-6481143-3-8 CC BY 3.0. Cataloguing data Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee -

Biosecurity Risk Assessment
An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries RIRDC Publication No. 11/141 RIRDCInnovation for rural Australia An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries by Dr Robert C Keogh February 2012 RIRDC Publication No. 11/141 RIRDC Project No. PRJ-007347 © 2012 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978-1-74254-320-8 ISSN 1440-6845 An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries Publication No. 11/141 Project No. PRJ-007347 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. -

Aphid Transmission of Potyvirus: the Largest Plant-Infecting RNA Virus Genus
Supplementary Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus Kiran R. Gadhave 1,2,*,†, Saurabh Gautam 3,†, David A. Rasmussen 2 and Rajagopalbabu Srinivasan 3 1 Department of Plant Pathology and Microbiology, University of California, Riverside, CA 92521, USA 2 Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC 27606, USA; [email protected] 3 Department of Entomology, University of Georgia, 1109 Experiment Street, Griffin, GA 30223, USA; [email protected] * Correspondence: [email protected]. † Authors contributed equally. Received: 13 May 2020; Accepted: 15 July 2020; Published: date Abstract: Potyviruses are the largest group of plant infecting RNA viruses that cause significant losses in a wide range of crops across the globe. The majority of viruses in the genus Potyvirus are transmitted by aphids in a non-persistent, non-circulative manner and have been extensively studied vis-à-vis their structure, taxonomy, evolution, diagnosis, transmission and molecular interactions with hosts. This comprehensive review exclusively discusses potyviruses and their transmission by aphid vectors, specifically in the light of several virus, aphid and plant factors, and how their interplay influences potyviral binding in aphids, aphid behavior and fitness, host plant biochemistry, virus epidemics, and transmission bottlenecks. We present the heatmap of the global distribution of potyvirus species, variation in the potyviral coat protein gene, and top aphid vectors of potyviruses. Lastly, we examine how the fundamental understanding of these multi-partite interactions through multi-omics approaches is already contributing to, and can have future implications for, devising effective and sustainable management strategies against aphid- transmitted potyviruses to global agriculture. -

Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth
International Journal of Molecular Sciences Article Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth Hong Chang 1,2, Jianglong Guo 3, Xiaowei Fu 4, Yongqiang Liu 2, Kris A. G. Wyckhuys 2, Youming Hou 1,* and Kongming Wu 2,* ID 1 State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops and Fujian Province Key Laboratory of Insect Ecology, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] 2 State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China; [email protected] (Y.L.); [email protected] (K.A.G.W.) 3 College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, China; [email protected] 4 Department of Plant Protection, Henan Institute of Science and Technology, Xinxiang 453003, China; [email protected] * Correspondence: [email protected] (Y.H.); [email protected] (K.W.); Tel.: +86-10-8210-5551 (K.W.) Received: 18 January 2018; Accepted: 8 February 2018; Published: 13 February 2018 Abstract: Pollen grains are regularly used as markers to determine an insect’s movement patterns or host (plant) feeding behavior, yet conventional morphology-based pollen grain analysis (or palynology) encounters a number of important limitations. In the present study, we combine conventional analytical approaches with DNA meta-barcoding to identify pollen grains attached to migrating adults of the turnip moth, Agrotis segetum (Lepidoptera: Noctuidae) in Northeast China. More specifically, pollen grains were dislodged from 2566 A. segetum long-distance migrants captured on Beihuang Island (Bohai Sea) and identified to many (plant) species level.