(Sbn 128515) Pratt & Associate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
MASTER UPC June08
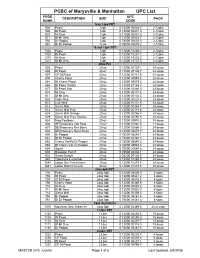
PCBC of Marysville & Manhattan UPC List PROD UPC DESCRIPTION SIZE PACK NUMB CODE 12oz 12pk PET 502 Pepsi 12pk 0 12000 00830 6 2-12pks 503 Dt Pepsi 12pk 0 12000 00831 3 2-12pks 510 Mt Dew 12pk 0 12000 00832 0 2-12pks 511 Dt Mt Dew 12pk 0 12000 00833 7 2-12pks 550 Dr Pepper 12pk 0 78000 00602 5 2-12pks 551 Dt Dr Pepper 12 pk 0 78000 00603 2 2-12pks 16.9oz 12pk PET 1302 Pepsi 12pk 0 12000 10200 4 2-12pks 1303 Dt Pepsi 12pk 0 12000 10201 1 2-12pks 1310 Mt Dew 12pk 0 12000 10203 5 2-12pks 1311 Dt Mt Dew 12pk 0 12000 10737 5 2-12pks 20oz Pet 602 Pepsi 20 oz 0 12000 00129 1 24 loose 603 Dt Pepsi 20 oz 0 12000 00130 7 24 loose 607 CF Dt Pepsi 20 oz 0 12000 00121 5 24 loose 690 Cherry Pepsi 20 oz 0 12000 00559 6 24 loose 691 Dt Cherry Pepsi 20 oz 0 12000 00579 4 24 loose 686 Dt Pepsi Vanilla 20 oz 0 12000 81189 0 24 loose 677 Dt Pepsi Max 20 oz 0 12000 01880 0 24 loose 610 Mt Dew 20 oz 0 12000 00131 4 24 loose 611 Dt Mt Dew 20 oz 0 12000 00134 5 24 loose 692 Code Red 20 oz 0 12000 00224 3 24 loose 618 Live Wire 20 oz 0 12000 81131 9 24 loose 613 Sierra Mist 20 oz 0 12000 00354 7 24 loose 612 Sierra Mist Free 20 oz 0 12000 20115 8 24 loose 628 Sierra Mist Orange 20 oz 0 12000 02786 4 24 loose 629 Sierra Mist Free Orange 20 oz 0 12000 02794 9 24 loose 634 Mug Rootbeer 20 oz 0 12000 00910 5 24 loose 636 DEWmocracy Volt Rasp 20 oz 0 12000 02862 5 24 loose 637 DEWmocracy Rev Berry 20 oz 0 12000 02866 3 24 loose 638 DEWmocracy Nova Straw 20 oz 0 12000 02870 0 24 loose 650 Dr Pepper 20 oz 0 78000 08240 1 24 loose 651 Dt Dr Pepper 20 oz 0 78000 08340 -

Bottles and Extras Fall 2006 44
44 Fall 2006 Bottles and Extras Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9 Fig. 10 Fig. 11 Fig. 12 Fig. 13 Fig. 14 Fig. 16 Fig. 17 Fig. 18 Fig. 19 Fig. 20 Fig. 21 Fig. 22 Fig. 23 Fig. 24 Fig. 25 Fig. 26 Fig. 27 Fig. 28 Fig. 29 Fig. 30 Fig. 31 Fig. 32 Fig. 33 Fig. 34 Fig. 35 Fig. 36 Fig. 37 Fig. 38 Fig. 39 Fig. 40 Fig. 42 Fig. 43 Fig. 45 Fig. 46 Fig. 47 Fig. 48 Fig. 52 Bottles and Extras March-April 2007 45 nationwide distributor of convenience– and dollar-store merchandise. Rosen couldn’t More Energy Drink Containers figure out why Price Master was not selling coffee. “I realized coffee is too much of a & “Extreme Coffee” competitive market,” Rosen said. “I knew we needed a niche.” Rosen said he found Part Two that niche using his past experience of Continued from the Summer 2006 issue selling YJ Stinger (an energy drink) for By Cecil Munsey Price Master. Rosen discovered a company named Copyright © 2006 “Extreme Coffee.” He arranged for Price Master to make an offer and it bought out INTRODUCTION: According to Gary Hemphill, senior vice president of Extreme Coffee. The product was renamed Beverage Marketing Corp., which analyzes the beverage industry, “The Shock and eventually Rosen bought the energy drink category has been growing fairly consistently for a number of brand from Price Master. years. Sales rose 50 percent at the wholesale level, from $653 million in Rosen confidently believes, “We are 2003 to $980 million in 2004 and is still growing.” Collecting the cans and positioned to be the next Red Bull of bottles used to contain these products is paralleling that 50 percent growth coffee!” in sales at the wholesale level. -

Brand/Package Line-Up
BRAND/PACKAGEBRAND/PACKAGE LINELINE ----UPUPUPUP Energy Drinks 16oz CANS (12pk) Carbonated Soft Drinks (CSD’s) Waters 20oz Bttls(24pk) Mountain Dew AMP 20oz BOTTLES (24pk) Aquafina Mountain Dew SF AMP 20oz BOTTLES (24pk) Amp Green Tea / Amp Black Tea 12oz CANS (24pk) Flavor Splash Grape AMP Lightning Lemonade +SF 2 Liter BOTTLES (8pk) Flavor Splash Lemon Iced Tea w/Lemon Green Tea w/Citrus AMP Sugar Free Lightning Lemonade Pepsi Flavor Splash Strawberry Kiwi Mountain Dew AMP Overdrive (Cherry) Diet Green Tea w/Citrus Diet Pepsi Flavor Splash Rasberry Mountain Dew AMP Revive (Orange Citrus) White Tea w/Raspberry Diet Pepsi MAX Flavor Splash Wildberry Mountain Dew AMP Maintain (Grape) Iced Tea Lemonade Mountain Dew AMP Focus (Mixed Berry) Cease Fire Max (Diet) Flavor Splash Peach Mango 1 Liter BOTTLES (12pk) Sparkling Green Tea Berry/Strbry Kiwi Sobe No Fear Caffeine Free Diet Pepsi Aquafina 20oz BOTTLES (24pk) Sobe SF No Fear 12oz CANS (24pk) Sobe No Fear Bloodshot (Org Dragonfruit) Caffeine Free Pepsi Brisk: Tea, Sweet Tea, Ras Tea, 2 Liter BOTTLES (8pk) Sobe No Fear Motherload Wild Cherry Pepsi Juicy Peach,Tea Lemonade, Fruit Punch ROCKSTAR ENERGY DRINKS Diet Wild Cherry Pepsi and Strawberry Melon Orangeade 16oz Can (24pk) Pepsi Throwback SOBE LW 20oz Bttls (12pk) Lemonade RockStar Energy Mountain Dew Throwback Yumberry Pomegranate 0Cal RockStar JCD GVA Black and Blueberry 0Cal Pink Lemonade Mountain Dew Strawberry Melon RockStar JCD MGO ORG Diet Mountain Dew Fuji Apple Pear 0Cal RockStar JCD POM Acai Fruit Punch 0Cal RockStar PNCHD Mountain Dew Code Red Mango Melon 0Cal RockStar PNCHD CITRUS Mountain Dew Live Wire Strawberry Dragon 0cal RockStar SF / Zero Carb Mountain Dew Voltage Raz Cherimoya Punch 0cal RockStar Recovery / Cola Sierra Mist Blackberry-Grape 15oz Cans (24pk) 20oz (NEW PET) BOTTLES (12pk) RockStar RSTD LTE Diet Sierra Mist Pomegranate-Cherry Green Tea Citrus Energy RockStar RSTD LT VAN Dr. -

U.S. Brands Shopping List
Breakfast Bars/Granola Bars: Other: Snacks Con’t: Quaker Chewy Granola Bars Aunt Jemima Mixes & Syrups Rold Gold Pretzels Quaker Chewy Granola Cocoa Bars Quaker Oatmeal Pancake Mix Ruffles Potato Chips Quaker Chewy Smashbars Rice Snacks: Sabra hummus, dips and salsas Quaker Dipps Granola Bars Quaker Large Rice Cakes Sabritones Puffed Wheat Snacks Quaker Oatmeal to Go Bars Quaker Mini Delights Santitas Tortilla Chips Quaker Stila Bars Quaker Multigrain Fiber Crisps Smartfood Popcorn Coffee Drinks: Quaker Quakes Smartfood Popcorn Clusters Seattle’s Best Coffee Side Dishes: Spitz Seeds Starbucks DoubleShot Near East Side Dishes Stacy’s Pita and Bagel Chips Starbucks Frappuccino Pasta Roni Side Dishes SunChips Multigrain Snacks Starbucks Iced Coffee Rice‐A‐Roni Side Dishes Tostitos Artisan Recipes Tortilla Chips Energy Drinks: Snacks: Tostitos Multigrain Tortilla Chips U.S. Brands AMP Energy Baked! Cheetos Snacks Tostitos Tortilla Chips No Fear Energy Drinks Baked! Doritos Tortilla Chips Soft Drinks: Shopping List Starbucks Refreshers Baked! Lay’s Potato Crisps Citrus Blast Tea/Lemonade: Baked! Ruffles Potato Chips Diet Pepsi Brisk Baked! Tostitos Tortilla Chips Diet Mountain Dew Lipton Iced Tea Baken‐ets Pork Skins and Cracklins Diet Sierra Mist Lipton PureLeaf Cheetos Cheese Flavored Snacks Manzanita Sol SoBe Tea Chester’s Flavored Fries Mirinda Tazo Tea Chester’s Popcorn Mountain Dew Juice/Juice Drinks: Cracker Jack Candy Coated Popcorn Mug Soft Drinks AMP Energy Juice Doritos -

Energy Drinks and Health: a Brief Review of Their Effects and Consequences
Ciencias de la Conducta ©2012 Universidad Carlos Albizu González, Miranda-Massari, Rodríguez-Gómez, Ricart y 2012. Vol. 27 – Núm. 1, 23-34 San Juan, Puerto Rico Rodríguez-Pagán Energy Drinks and Health: A Brief Review of Abstract their Effects and Consequences Energy drinks have been promoted as a healthy beverage within many subpopulations, as for instance, athletes and college students. The chemical Composition of energy drinks can produce multiple adverse effects, including serious behavioral ones. This article presents and Michael J. González1, Jorge R. Miranda-Massari2, José discusses, in a brief form, some serious health complications of energy Rodríguez Gómez3, Carlos M. Ricart4 y Dharma Rodriguez- drinks on their human consumers. Pagán51 Keywords: Energy drinks effects, athletes, college students Resumen Las bebidas energizantes tan comúnmente promovidas como saludables en múltiples escenarios y subpoblaciones como lo son atletas y estudiantes universitarios pueden tener serios efectos adversos debido a sus ingredientes. Su consumo puede llevar a sufrir serias complicaciones incluyendo efectos adversos en el comportamiento. Este Energy drinks became popular in Asia long before they reached artículo presenta y discute, en forma breve, algunas implicaciones serias the United States. In 1962, the Japanese pharmaceutical company, que pueden presentar estas bebidas, muy particularmente en términos de Taisho, released its Lipovitan D drink. It was designed to help salud. employees to work hard well into the night hours. An Austrian businessman, Dietrich Mateschitz picked up on the business potential of energy drinks while on a trip to Asia. Along with two Thai business Palabras Claves: Bebidas energizantes, atletas, estudiantes partners, Mateschitz started the company Red Bull GmbH, with the idea universitarios of marketing the drink to young Europeans. -

2007 Annual Report
Common Stock Information Shareholder Information PepsiCo Stock Purchase Program — for Canadian employees: Fidelity Stock Plan Services Stock Trading Symbol — PEP Annual Meeting P.O. Box 5000 Contents Stock Exchange Listings The Annual Meeting of Shareholders will be held at Frito-Lay Cincinnati, OH 45273-8398 The New York Stock Exchange is the principal market for Corporate Headquarters, 7701 Legacy Drive, Plano, Texas, Telephone: 800-544-0275 1 ...... Financial Highlights PepsiCo common stock, which is also listed on the Chicago on Wednesday, May 7, 2008, at 9:00 a.m. local time. Website: www.iStockPlan.com/ESPP and Swiss Stock Exchanges. Proxies for the meeting will be solicited by an independent Please have a copy of your most recent statement available 2 ...... Letter to Shareholders Shareholders proxy solicitor. This Annual Report is not part of the proxy when calling with inquiries. solicitation. 7 ...... Questions & Answers As of February 8, 2008, there were approximately 185,000 10..... Leadership Team shareholders of record. Inquiries Regarding Your Stock Holdings If using overnight or certified mail send to: Fidelity Investments 12..... PepsiCo Americas Foods Dividend Policy Registered Shareholders (shares held by you in your name) should address communications concerning transfers, state- 100 Crosby Parkway 14..... PepsiCo Americas Beverages We target an annual dividend payout of 50% of prior year’s ments, dividend payments, address changes, lost certificates Mail Zone KC1F-L earnings, excluding certain items. Dividends are usually 16..... PepsiCo International and other administrative matters to: Covington, KY 41015 19..... Purpose: Human, Environment, Talent declared in late January or early February, May, July and November and paid at the end of March, June and 29.... -

Appendix Unilever Brands
The Diffusion and Distribution of New Consumer Packaged Foods in Emerging Markets and what it Means for Globalized versus Regional Customized Products - http://globalfoodforums.com/new-food-products-emerging- markets/ - Composed May 2005 APPENDIX I: SELECTED FOOD BRANDS (and Sub-brands) Sample of Unilever Food Brands Source: http://www.unilever.com/brands/food/ Retrieved 2/7/05 Global Food Brand Families Becel, Flora Hellmann's, Amora, Calvé, Wish-Bone Lipton Bertolli Iglo, Birds Eye, Findus Slim-Fast Blue Band, Rama, Country Crock, Doriana Knorr Unilever Foodsolutions Heart Sample of Nestles Food Brands http://www.nestle.com/Our_Brands/Our+Brands.htm and http://www.nestle.co.uk/about/brands/ - Retrieved 2/7/05 Baby Foods: Alete, Beba, Nestle Dairy Products: Nido, Nespray, La Lechera and Carnation, Gloria, Coffee-Mate, Carnation Evaporated Milk, Tip Top, Simply Double, Fussells Breakfast Cereals: Nesquik Cereal, Clusters, Fruitful, Golden Nuggets, Shreddies, Golden Grahams, Cinnamon Grahams, Frosted Shreddies, Fitnesse and Fruit, Shredded Wheat, Cheerios, Force Flake, Cookie Crisp, Fitnesse Notes: Some brands in a joint venture – Cereal Worldwide Partnership, with General Mills Ice Cream: Maxibon, Extreme Chocolate & Confectionery: Crunch, Smarties, KitKat, Caramac, Yorkie, Golden Cup, Rolo, Aero, Walnut Whip, Drifter, Smarties, Milkybar, Toffee Crisp, Willy Wonka's Xploder, Crunch, Maverick, Lion Bar, Munchies Prepared Foods, Soups: Maggi, Buitoni, Stouffer's, Build Up Nutrition Beverages: Nesquik, Milo, Nescau, Nestea, Nescafé, Nestlé's -

ENERGY DRINK CONTAINERS - Bottles & Cans by Cecil Munsey Copyright © 2006
52 Summer 2006 Bottles and Extras Fig. 1 Fig. 2 Fig. 3 Fig. 4 Fig. 5 Fig. 6 Fig. 7 Fig. 8 Fig. 9 Fig. 10 Fig. 11 Fig. 12 Fig. 13 Fig. 14 Fig. 15 Fig. 16 Fig. 17 Fig. 18 Fig. 19 Fig. 20 Fig. 21 Fig. 22 Fig. 23 Fig. 24 Fig. 25 Fig. 30 Fig. 31 Fig. 26 Fig. 27 Fig. 28 Fig. 29 Fig. 32 > < Fig. 33 Fig. 36 < Fig. 37 Fig. 35 Fig. 38 Fig. 34 > Bottles and Extras Summer 2006 53 ENERGY DRINK CONTAINERS - Bottles & Cans By Cecil Munsey Copyright © 2006 AUTHOR’S NOTE: This article can save you from making a mistake similar to one I made 35+ years ago. I look back now with melancholy at the time while completing the manuscript of my popular study of the Coca-Cola Company’s merchandising history – “The Illustrated Guide to the Collectibles of Cola” (Hawthorn Books, NY). – I consciously did not include a chapter on Coca- Cola cans. Why? I didn’t think that collectors would be interested in “rusty, old tin cans.” I overlooked the entire category except for a few of the early cone-top models and didn’t take into account the successful introduction of aluminum cans and bottles as attractive and long-lasting beverage containers. This article doesn’t make any such mistake; it glorifies a whole, relatively new, category of collectible containers, thus giving collectors the head-start information needed to begin a collection of energy drink cans and bottles. Thanks to eBay and other sources, the current beverages and even those products that didn’t make it in the fast- paced energy drink market, are still available. -

UMC Preferred Vendors Direct from UMC Orders Quantity Pepsi Products
Customer Name Team Name E-mail Phone UMC Preferred Vendors Generators, Gators, UTVs, Machinery Golf Cars Tools and Shop Supplies Western States Equipment Intermountain Golf Cars Industrial Supply Jim Parker Jason Short Located In Store On Site In UMC Paddock Office: 801-596-2300 Office: 801-255-8828 Industrial Supply can quickly get most any Cell: 385-235-1807 Cell: 801-918-3207 tool or supply need delivered onsite within [email protected] [email protected] less than 24 hours. Direct from UMC orders Contact Derrek Degraaff directly to place order 435-578-0352 Or e-mail order & questions to - [email protected] Item Quantity Cinder Blocks - 1000 lbs - $100 Per Block Pepsi Products (See Attached Product and Price Sheet) Pepsi products will be stored at our kart center and available for you to pick up when you arrive. Quantity Order must be placed 1 week in advance. Order is per case List Products Here Total Order Amount $ Tickets and Hospitality—Contact Derrek Degraaff Directly for Group Ticket Rates and Hospitality and Menu Options - 435-578-0352 or [email protected] Garages and RV Spaces For Garage Or Extra RV Space Contact Vicki Zollinger at the UMC Box office - 435-216-5565 or [email protected] Beverage Pricing Sheet Sodas 12 Oz Cans, 24 Cans Per Case - $12.00 Per Case 20 Oz Plastic Bottles , 24 Bottles Per Case - $25.00 Per Case Water Aquafina - 12 Oz Plastic Bottles, 24 Bottles Per Case - Aquafina - 20 Oz Plastic Bottles, 24 Bottles Per Case - $15.00 Per Case Gatorade & G2 20 Oz Plastic Bottles, 24 Bottles Per Case - $25.00 Per Case Energy Drinks AMP & Rockstar Energy Drinks - 16 Oz Cans, 12 Cans Per Case - $22.00 Per Case Mountain Dew Kick Start - 16 Oz Cans, 12 cans per Case - $22.00 Per Case Xyience Energy Drinks - 16 Oz Cans, 12 Cans Per Case - $20.00 Per Case Other Specialty Drinks Other beverages such as: Sobe, Starbucks, Ocean Spray are also available for purchase, see order form 2 for per case pricing. -

Barr's Vending Vending Product List
Barr's Vending Vending Product List Pepsi Products Candy Chips & Snacks Caffeine Free Diet Pepsi Almond Joy Baked Cheddar Snack Mix Caffeine Free Pepsi Baby Ruth Beef & Cheese Sticks Diet Dew Butterfinger Beef & Cheese Sticks Diet Green Tea Butterfinger Crisp Bugles Diet Pepsi Caramel Creams Cheetos Crunchy Diet Sierra Mist Cookies N Cream Cheez-it Diet Wild Cherry Pepsi Hershey's Almond Chex Mix Gingerale Kit-Kat Combos Grape Crush M&M's Peanut Crunch N Munch Green Tea With Citrus M&M's Plain Dorito's Cool Ranch Lipton Tea Mallo Cup Dorito's Nacho Mountain Dew Milky Way Frito's Mountain Dew Code Red Mounds Frito's Chili Cheese Mountain Dew Live Wire Nestle Crunch Gardettos Mug Root Beer Nutrageous Goldfish Orange Crush Payday Hickory Smoked Beef Sticks Pepsi Peanut Bar Hot Fries Sierra Mist Raisinet Jalapeno Cheese Curls Wild Cherry Pepsi Reese's Peanut Butter Cup Kellog's Pop-Tarts Skittles Lance Crackers Aguafina Waters Snickers Lays Classic Aguafina Swedish Fish Lays KC Masterpiece Splash Citrus Take 5 Lays Salt & Vinegar Splash Grape Three Musketeers Lays Sour Cream & Onion Splash Kiwi Twix Micro Popcorn Butter Lovers Splash Lemon Twizzlers Micro Popcorn Light Butter Splash Peach Whatchamacallit Peanut Bar Splash Raspberry York Peppermint Patty Popcorn White Cheddar Splash Wildberry And many more... Ramon Soups Ritz Cheddar Crackerfuls Sobe Life Waters Pastries/Cookies Ruffles Cheddar & Sr Cream Cherry Apple Pie Slim Jim Fruit Citrus Big Texas Cinnamon Snyders BBQ Grape - Blackberry Cherry Pie Wheat Thins Orange - Tangerine Coffee -

³Brand Promotion of Tzinga Energy Drink
³Brand Promotion Of Tzinga Energy Drink´ Summer Internship Project Report Submitted towards Partial Fulfillment of Post Graduate Diploma in Management-MM (Approved by AICTE, Govt. of India) Academic Session 2010 ± 2012 Submitted By: Dheeraj Singh Somvanshi Roll No ± BM.010262 Under the Guidance of: Industry Guide: Faculty Guide: Mr. Harsingh sir prof UrvashiMakkar Designation Designation: facultyHector Beverages Pvt. Ltd.IMS, Ghaziabad INSTITUTE OF MANAGEMENT STUDIES GHAZIABAD A Project Report Of Summer Internship Project on ³Brand Promotion Of Tzinga Energy Drink´ Submitted To:Submitted By: Prof UrvashiMakk Dheeraj Singh Somvanshi BM 010262 DECLARATION I, Dheeraj Singh Somvanshi, BM-010262 here by declared that the work which is being presented in the report etitled ³Brand Promotion of Tzinga energy Drink´ in partial fulfillment of the requirement for the award of Post Graduate Diploma in Management studies, Ghaziabad is an authentic record of our own work carried out during period from April 2010 to july 2010 under the supervision of Mr. Harsingh (ASM) and Ms. UJwalla (HR, Hector Gurgaon) and regular guidance by Prof. Urvashi Makkar(Faculty Mentor, IMS Ghaziabad). I have not submitted the matter presented in the report for the award of any other degree or diploma of this or any other institute. Date: July 15th , 2010 Dheeraj Singh Somvanshi Place: Ghaziabad PREFACE The PGDM program is well structured and integrated course of business studies. The main objective of practical training at PGDM level is to develop skill in student by supplement to the theoretical study of business management in general. Industrial training helps to gain real life knowledge about the industrial environment and business practices. -

Pepsi Products
Pepsi Products 12 OZ CARBONATED AND NON-CARBONATED 20 OZ CARBONATED BEVERAGES PEPSI COLA PEPSI WILD CHEERY CHERRY PEPSI SIERRA MIST CRANBERRY SPLASH CAFFEINE FREE PEPSI PEPSI MAX PEPSI ONE PEPSI COLA PEPSI THROWBACK DIET PEPSI PEPSI MAX MOUNTAIN DEW PEPSI WILD CHERRY DIET MOUNTAIN DEW DIET PEPSI MOUNTAIN DEW VOLTAGE DIET CAFFEINE FREE PEPSI DIETT SIERRA MIST CRANBERRY SPLASH DIET PEPSI LIME SIERRA MIST DIET PEPSI WILD CHERRY MOUNTAIN DEW CODE RED MOUNTAIN DEW DIET PEPSI VANILLA MOUNTAIN DEW CODE RED DIET PEPSI LIME MOUNTAIN DEW LIVE WIRE DR. PEPPER MOUNTAIN DEW VOLTAGE DIET DR. PEPPER MOUNTAIN DEW THROWBACK MUG RT BEER MOUNTAIN DEW GAME FUEL CITRUS CHERRY DIET SIERRA MIST MOUNTAIN DEW GAME FUEL WILD FRUIT MOUNTAIN DEW LIVE WIRE CAFFEINE FREE MOUNTAIN DEW DIET PEPSI WILD CHERRY DIET CAFFEINE FREE MOUNTAIN DEW DIET CAFFEINE PEPSI DIET MOUNTAIN DEW DR. PEPPER CHERRY DIET MOUNTAIN DEW ULTRA VIOLET DIET DR. PEPPER CHERRY DIET MOUNTAIN DEW CODE RED SIERRA MIST RUBY SPLASH DR. PEPPER SIERRA MIST DIET RUBY SPLASH DR. PEPPER CHERRY CRUSH ORANGE DIET DR. PEPPER DIET CRUSH ORANGE DIET DR. PEPPER CHERRY CRUSH GRAPE DIET DR. PEPPER VANILLA CRUSH STRAWBERRY DIET CAFFEINE FREE DR. PEPPER CRUSH PINEAPPLE CAFEINE FREE DR. PEPPER CRUSH PEACH LIPTON BRISK SWEETENED W/LEMON PEPSI THROWBACK LIPTON BRISK GREEN TEA MOUNTAIN DEW THROWBACK LIPTON BRISK SWEETENED WITHOUT LEMON MOUNTAIN DEW GAME FUEL CITRUS CHERRY DIET LIPTON BRISK UNSWEETENED W/LEMON MOUNTAIN DEW GAME FUEL WILD FRUIT MUG ROOT BEER DIET MOUNTAIN DEW ULTRA VIOLET DIET MUG ROOT BEER SIERRA MIST 20