The Introduction of Diphtheria-Tetanus-Pertussis And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Double-Sided Effects of Mycobacterium Bovis Bacillus Calmette–Guérin Vaccine ✉ ✉ Junli Li 1,2,3,4, Lingjun Zhan1,2,3,4 and Chuan Qin 1,2,3,4
www.nature.com/npjvaccines REVIEW ARTICLE OPEN The double-sided effects of Mycobacterium Bovis bacillus Calmette–Guérin vaccine ✉ ✉ Junli Li 1,2,3,4, Lingjun Zhan1,2,3,4 and Chuan Qin 1,2,3,4 Bacillus Calmette–Guérin (BCG), the only vaccine proven to be effective against tuberculosis (TB), is the most commonly used vaccine globally. In addition to its effects on mycobacterial diseases, an increasing amount of epidemiological and experimental evidence accumulated since its introduction in 1921 has shown that BCG also exerts non-specific effects against a number of diseases, such as non-mycobacterial infections, allergies and certain malignancies. Recent Corona Virus Disease 2019 (COVID-19) outbreak has put BCG, a classic vaccine with significant non-specific protection, into the spotlight again. This literature review briefly covers the diverse facets of BCG vaccine, providing new perspectives in terms of specific and non-specific protection mechanisms of this old, multifaceted, and controversial vaccine. npj Vaccines (2021) 6:14 ; https://doi.org/10.1038/s41541-020-00278-0 INTRODUCTION infections observed in children after receiving BCG vaccina- 17–20 Bacillus Calmette–Guérin (BCG), a live-attenuated bacterial tion . Clinical evidence also suggests that BCG may be 1234567890():,; effective against infections caused by viral pathogens, such as vaccine derived from Mycobacterium bovis was originally – 1 respiratory syncytial virus21,22, human papilloma virus23 25,and isolated in 1902 from a cow with tuberculosis (TB) .Theisolate 26 was cultured continuously for >230 generations for 13 years herpes simplex virus . Moreover, an increasing number of (1908–1921) to generate a mutant strain with weakened animal studies using mouse models have demonstrated the virulence but with high immunogenicity2. -

BCG Coverage and Barriers to BCG Vaccination In
Thysen et al. BMC Public Health 2014, 14:1037 http://www.biomedcentral.com/1471-2458/14/1037 RESEARCH ARTICLE Open Access BCG coverage and barriers to BCG vaccination in Guinea-Bissau: an observational study Sanne Marie Thysen1,2*, Stine Byberg1,2, Marie Pedersen1,2, Amabelia Rodrigues1, Henrik Ravn2,3, Cesario Martins1, Christine Stabell Benn2,3, Peter Aaby1,2,3 and Ane Bærent Fisker1,2 Abstract Background: BCG vaccination is recommended at birth in low-income countries, but vaccination is often delayed. Often 20-dose vials of BCG are not opened unless at least ten children are present for vaccination (“restricted vial-opening policy”). BCG coverage is usually reported as 12-month coverage, not disclosing the delay in vaccination. Several studies show that BCG at birth lowers neonatal mortality. We assessed BCG coverage at different ages and explored reasons for delay in BCG vaccination in rural Guinea-Bissau. Methods: Bandim Health Project (BHP) runs a health and demographic surveillance system covering women and their children in 182 randomly selected village clusters in rural Guinea-Bissau. BCG coverage was assessed for children born in 2010, when the restricted vial-opening policy was universally implemented, and in 2012–2013, where BHP provided BCG to all children at monthly visits in selected intervention regions. Factors associated with delayed BCG vaccination were evaluated using logistic regression models. Coverage between intervention and control regions were evaluated in log-binomial regression models providing prevalence ratios. Results: Among 3951 children born in 2010, vaccination status was assessed for 84%. BCG coverage by 1 week of age was 11%, 38% by 1 month, and 92% by 12 months. -

EPI-NEWS NATIONAL SURVEILLANCE of COMMUNICABLE DISEASES Editor: Peter Henrik Andersen Dept
EPI-NEWS NATIONAL SURVEILLANCE OF COMMUNICABLE DISEASES Editor: Peter Henrik Andersen Dept. of Epidemiology Tel.: +45 3268 3268 • Fax: +45 3268 3874 Statens Serum Institut • 5 Artillerivej • DK 2300 Copenhagen S www.ssi.dk • [email protected] • ISSN: 1396-4798 DANISH HEALTH RESEARCH IN GUINEA-BISSAU No. 34, 2006 In one of the world’s poorest years, the BHP has been the focus of yields 70% protection against countries, Guinea-Bissau in West 22 PhD theses, mainly Danish, and rotavirus diarrhoea, and 52% Africa, the Bandim Health Project nine doctoral dissertations. protection against reinfection during (BHP) has been engaged in epide- the first year following the initial miological research since 1978 Research focus areas infection. This high protection rate focussing on infection, vaccination Two-dose measles vaccination suggests that a rotavirus vaccine and the long-term effects of health strategy: would be an effective means to intervention. Infection with measles before the reduce the incidence of acute The BHP is a collaboration between WHO recommended vaccination age diarrhoea with dehydration and Statens Serum Institut and the at 9 months is a growing problem in associated deaths. Guinea-Bissau Ministry of Health. the developing countries. This is For further information on the BHP, Child mortality in Guinea-Bissau due, among others, to increasing please contact project secretary ranks among the highest worldwide: urbanisation and an increase in the Christina Rasmussen, [email protected]. > 200 per 1000 live born infants share of mothers who were them- (P. Valentiner-Branth, K. Mølbak, during the first three years of life. selves measles vaccinated at a young Dept. -
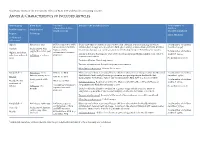
Annex A: Characteristics of Included Articles
Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines ANNEX A: CHARACTERISTICS OF INCLUDED ARTICLES Article group Birth dates Vaccines Details of the included article Total number of administered (type, children Ref ID (citation) Study period strain, reason) described/analysed Region Follow up Effect Modifier (Additional references)1 Algeria Birth dates: 1935 BCG vaccinated vs. BCG Quasi-randomised controlled trial conducted in a Muslim population in Algeria where Total number of children unvaccinated (Institute children born in 1935 were allocated to BCG (given orally) or unvaccinated at birth. Children described: 41,307 #9283(1) Study period: May Pasteur, orally revaccinated at ages 1, 3, 7 and 15 years were followed up for up to 6 months after vaccine. 1935 to December 1947 Total number of children Algiers, periurban, administered, research Inclusion Criteria: Participants were newborns from Algerian Muslim families with very low analysed: 39,259 suburban and rural Follow up: ≤ 12 years purpose) socioeconomic status. areas No modifier reported Exclusion Criteria: Not clearly stated Vaccine ascertainment: Recorded at point of vaccination Mortality ascertainment: Routine home visits Bangladesh A Birth dates: born BCG vs. no BCG Observational cohort reporting all-cause child mortality from 70 villages within the Maternal Total number of children before 1 January 2000 Child Health And Family Planning programme area participating in the Health And described: 39,625 #797(2) DPT vs. no DPT Demographic Surveillance -

Too Many Chefs in Africa Blocked Completely
ORIGINAL ARTICLE Yet, uncertain data like this forms basis for donations to the health sector, and DC that are not able to demonstrate a reduction in child mortality will experience that donations are withheld or are Too many chefs in Africa blocked completely. Nevertheless, data from longitudinal demo- graphic health surveillance research sites in DC follow child popula- – secondary publication tions that are large enough to capture even smaller changes in child mortality [2]. Morten Sodemann, Senior Researcher On the contrary, donors have excluded long-term involvement with rapid diminishing resources for development aid, demands for short-term goals and lacking support of valid mortality data [3]. In Statens Serum Institut, Bandim Health project. an editorial in The Lancet 2004 it is concluded that biomedical re- Correspondence: Morten Sodemann, Bandim Health project, Statens Serum search has failed to tackle the massive health problems in DC [4]. Institut, Artellerivej 5, 2300 København S, Denmark. E-mail: [email protected] ABSTINENCE AND RELUCTANCE The 2015 goals are a political manifest and should be seen in that Dan Med Bull 2007;54:52-4 context. There are urgent health problems that are not mentioned in the millennium goals. The significance of persistently low quality of ABSTRACT care in DC health sectors as well as the poor dissemination of exist- Available interventions could prevent six out of ten million deaths in chil- ing well proven health interventions like child immunizations are dren younger than five years of age every year. The health sector and donor examples of this. A decline in maternal mortality is bound up with agencies bear responsibility for not using these interventions. -

UNICEF GUINEA-BISSAU Review of Malnutrition Prevention and Management Project
UNICEF GUINEA-BISSAU Review of Malnutrition Prevention and Management Project March 2018 Table of Contents Table of Figures ............................................................................................................................................................. 1 Executive Summary ....................................................................................................................................................... 2 Acknowledgements ....................................................................................................................................................... 4 Introduction ................................................................................................................................................................... 5 Objectives ...................................................................................................................................................................... 5 Key definitions ............................................................................................................................................................... 5 Background and Context ............................................................................................................................................... 6 Evaluation ...................................................................................................................................................................... 8 Challenges and Suggestions for the Future ................................................................................................................ -

Promotion of Exclusive Breastfeeding Is Not Likely to Be Cost Effective in West Africa
Acta Pædiatrica ISSN 0803–5253 REGULAR ARTICLE Promotion of exclusive breastfeeding is not likely to be cost effective in West Africa. A randomized intervention study from Guinea-Bissau Marianne S. Jakobsen ([email protected]), Morten Sodemann, Sidu Biai, Jens Nielsen, Peter Aaby Bandim Health Project, Indepth Network, Danish Epidemiology Science Centre, Apartado 861, Bissau, Guinea-Bissau; and Statens Serum Institut, 2300 Copenhagen S, Denmark Keywords Abstract Anthropometry, Epidemiological study, Exclusive Aim: To evaluate the impact of promotion of exclusive breastfeeding on infant health in Guinea- breastfeeding, Guinea-Bissau, Infant mortality and morbidity, Randomized intervention study Bissau, West Africa, where mortality rates are high, breastfeeding is widely practiced but exclusive Correspondence breastfeeding is rare. Marianne Skytte Jakobsen, Bandim Health Project, Method: At the Bandim Health Project in Guinea Bissau, West Africa, a birth cohort of 1721 infants Danish Epidemiology Science Centre, Statens were randomized to receive health education: promotion of exclusive breastfeeding for the first Serum Institut 2300, Copenhagen S, Denmark. Email: [email protected] 4–6 months of life according to WHO recommendations at the time of the study. All children were followed from birth to 6 months of age. Received 2 April 2007; revised 2 June 2007; Results: Introduction of both water and weaning food was significantly delayed in the intervention accepted 23 August 2007 group. However we found no beneficial health effects of the intervention; there was no reduction in DOI:10.1111/j.1651-2227.2007.00532.x mortality in the intervention group compared with the control group (mortality rate ratio: 1.86 (0.79–4.39)), weight at 4–6 months of age was significantly lower in the intervention group (7.10 kg vs. -

Study Protocol and Statistical Analysis Plan
BCG-DENMARK-COVID Protocol version 5.3, 20-04-2021 EudraCT: 2020-001888-90 Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic. A randomized controlled multi-center trial. (BCG-DENMARK-COVID) Protocol version 5.3, 20-04-2021 EudraCT: 2020-001888-90 1 BCG-DENMARK-COVID Protocol version 5.3, 20-04-2021 EudraCT: 2020-001888-90 PROTOCOL SIGNATURE SHEET Name Signature Date Sponsor: Christine Stabell Benn, MD, Prof. University of Southern Denmark 20-04-2021 Clinical Institute, OPEN Bandim Health Project Studiestræde 6, 1455 Copenhagen K Coordinating Principal Investigator: Anne Marie Rosendahl Madsen, MD University of Southern Denmark 20-04-2021 Clinical Institute, OPEN Heden 16, 5000 Odense C 2 BCG-DENMARK-COVID Protocol version 5.3, 20-04-2021 EudraCT: 2020-001888-90 Using BCG vaccine to enhance non-specific Protocol title protection of health care workers during the COVID-19 pandemic. A randomized controlled multi-center trial. Protocol ID BCG-DENMARK-COVID Short title BCG vaccine for healthcare workers against COVID-19 EudraCT number 2020-001888-90 Version 5.3 Date 20-04-2021 Sponsor Christine Stabell Benn OPEN Department of Clinical Research, University of Southern Denmark Principal Investigator (PI) Anne Marie Rosendahl Madsen, MD, PhD student OPEN Department of Clinical Research, University of Southern Denmark Investigator Frederik Schaltz-Buchholzer, MD, PhD Fellow Department of Clinical Research, University of Southern Denmark Collaborators at participating hospitals: Odense Universityhospital, Odense Isik Somuncu Johansen, Dep. of Infectious Diseases, and Svendborg Odense. Hvidovre/Amager/Glostrup Hospital Thomas Lars Benfield, Dep. of Infectious Diseases, Hvidovre. -

HTLV-1 and HIV-2 Infection Are Associated with Increased Mortality in a Rural West African Community
This is a repository copy of HTLV-1 and HIV-2 Infection Are Associated with Increased Mortality in a Rural West African Community. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/141771/ Version: Published Version Article: van Tienen, C., van der Loeff, M.S., Peterson, I et al. (8 more authors) (2011) HTLV-1 and HIV-2 Infection Are Associated with Increased Mortality in a Rural West African Community. PLoS ONE , 6 (12). e29026. ISSN 1932-6203 https://doi.org/10.1371/journal.pone.0029026 Reuse This article is distributed under the terms of the Creative Commons Attribution (CC BY) licence. This licence allows you to distribute, remix, tweak, and build upon the work, even commercially, as long as you credit the authors for the original work. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ HTLV-1 and HIV-2 Infection Are Associated with Increased Mortality in a Rural West African Community Carla van Tienen1, Maarten Schim van der Loeff2*, Ingrid Peterson1, Matthew Cotten1,So¨ ren Andersson3, Birgitta Holmgren4, Tim Vincent1, Thushan de Silva1,5, Sarah Rowland-Jones6, Peter Aaby7, Hilton Whittle1 1 Medical Research Council, Fajara, The Gambia, 2 Municipal Health Service and Academic Medical Centre, -

Location of the Bandim HDSS Centre, Guinea-Bissau
G U I N E A - B I S S A U Guinea-BissauGuinea-Bissau BissauBissau CityCity BissauBissau CityCity BissauBissau CityCity BandimBandim DSSDSS areaarea 00 2.52.5 55 00 5050 100100 KilometreKilometre KilometreKilometre Location of the Bandim HDSS centre, Guinea-Bissau The Bandim Health Project (http:// www.bandim.org ) was initiated in 1978 with the aim of improving child survival in Guinea Bissau. The Bandim Health Project is part of the National Institute of Health (INASA) in Guinea-Bissau and supported by a research group at the Statens Serum Institut, Copenhagen. The research station in Guinea-Bissau currently employs several Guinean physicians and epidemiologists involved in research training and a few expatriates, medical doctors, statistician, immunologist, medical and public health students. The field station registers the basic demographic and health-related data for approximately 102,000 inhabitants in the capital Bissau and 182 randomly selected clusters of villages are followed in the interior of Guinea-Bissau. More than 180 Guineans are employed for the continuous collection of surveillance data and for data collection linked to specific trials. Part of the research training, data analyses and writing of papers takes place at Statens Serum Institut, Copenhagen, and the potential implications of findings in Guinea-Bissau for Danish children are explored. Seven Guineans have obtained a PhD at University of Copenhagen. Objectives • To follow long-term consequences of various infections, health conditions and interventions. • To conduct research on determinants of measles mortality and evaluation of various measles vaccination schemes. • To study the non-specific immune training effects of vaccinations, vitamin A supplementation and other interventions. -

Out-Of-Sequence DTP and Measles Vaccinations and Child Mortality in Guinea-Bissau: a Reanalysis
Open access Original research BMJ Open: first published as 10.1136/bmjopen-2018-024893 on 5 September 2019. Downloaded from Out-of-sequence DTP and measles vaccinations and child mortality in Guinea-Bissau: a reanalysis Sanne M Thysen, 1,2,3,4 Amabelia Rodrigues,2 Peter Aaby,2,3 Ane B Fisker1,2,3 To cite: Thysen SM, ABSTRACT Strengths and limitations of this study Rodrigues A, Aaby P, et al. Objectives To assess whether the sequence of Out-of-sequence DTP and diphtheria-tetanus-pertussis vaccine (DTP) and measles ► Vaccination status of the children were only updated measles vaccinations and vaccine (MV) was associated with child survival in a child mortality in Guinea- at the inspection of a vaccination card. dataset previously used to assess non-specific effects of Bissau: a reanalysis. BMJ Open ► This study used the landmark analyses and thus vaccines with no consideration of vaccination sequence. 2019;9:e024893. doi:10.1136/ prevented survival bias. Design Prospective cohort study analysed using the bmjopen-2018-024893 ► Misclassification of vaccinations due to the land- landmark approach. mark approach would yield conservative estimates. ► Prepublication history and Setting Bandim Health Project’s Health and Demographic ► Booster doses of diphtheria-tetanus-pertussis (DTP) additional material for this Surveillance System covering 100 village clusters in rural paper are available online. To were not registered before 1996, and we were Guinea-Bissau. The recommended vaccination schedule view these files, please visit therefore not able to make any firm conclusions of was BCG and oral polio vaccine (OPV) at birth, DTP and the journal online (http:// dx. -

Seasonal Variation in the Non-Specific Effects
Original research Seasonal variation in the non- specific BMJ Glob Health: first published as 10.1136/bmjgh-2019-001873 on 5 March 2020. Downloaded from effects of BCG vaccination on neonatal mortality: three randomised controlled trials in Guinea- Bissau Kristoffer Jarlov Jensen,1,2 Sofie Biering- Sørensen,1 Johan Ursing,3,4 Poul- Erik Lund Kofoed,5,6 Peter Aaby,1,6 Christine Stabell Benn1,7 To cite: Jensen KJ, ABSTRACT Key questions Biering- Sørensen S, Ursing J, The BCG vaccine protects non- specifically against other et al. Seasonal variation in diseases than tuberculosis. Three randomised controlled What is already known? the non- specific effects of trials of early BCG in Guinea- Bissau found a 38% reduction BCG vaccination on neonatal BCG vaccine non- specifically protects against oth- in all- cause neonatal mortality. Little is known about the ► mortality: three randomised er diseases than tuberculosis presumably via its underlying mechanisms. In Guinea- Bissau, prevalent controlled trials in Guinea- immune-modula tory properties, training the innate infectious diseases display distinct seasonality. Revisiting Bissau. BMJ Global Health immune system to enhance response potential. 2020;5:e001873. doi:10.1136/ the three trials (>6500 infants) comparing early BCG ► Malaria in pregnancy is associated with an immune- bmjgh-2019-001873 versus no early BCG in low weight infants on all- cause compromised phenotype in the newborn and a high- neonatal mortality over 12 consecutive years, we explored er infant morbidity and mortality. Handling editor Seye Abimbola the seasonal variation in BCG’s effect on mortality. In a subgroup of participants, adaptive and innate cytokine What are the new findings? Received 27 July 2019 responses were measured 4 weeks after randomisation.