The Uricosuria and Orotic Aciduria Induced by 6- Azauridine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

35 Disorders of Purine and Pyrimidine Metabolism
35 Disorders of Purine and Pyrimidine Metabolism Georges van den Berghe, M.- Françoise Vincent, Sandrine Marie 35.1 Inborn Errors of Purine Metabolism – 435 35.1.1 Phosphoribosyl Pyrophosphate Synthetase Superactivity – 435 35.1.2 Adenylosuccinase Deficiency – 436 35.1.3 AICA-Ribosiduria – 437 35.1.4 Muscle AMP Deaminase Deficiency – 437 35.1.5 Adenosine Deaminase Deficiency – 438 35.1.6 Adenosine Deaminase Superactivity – 439 35.1.7 Purine Nucleoside Phosphorylase Deficiency – 440 35.1.8 Xanthine Oxidase Deficiency – 440 35.1.9 Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency – 441 35.1.10 Adenine Phosphoribosyltransferase Deficiency – 442 35.1.11 Deoxyguanosine Kinase Deficiency – 442 35.2 Inborn Errors of Pyrimidine Metabolism – 445 35.2.1 UMP Synthase Deficiency (Hereditary Orotic Aciduria) – 445 35.2.2 Dihydropyrimidine Dehydrogenase Deficiency – 445 35.2.3 Dihydropyrimidinase Deficiency – 446 35.2.4 Ureidopropionase Deficiency – 446 35.2.5 Pyrimidine 5’-Nucleotidase Deficiency – 446 35.2.6 Cytosolic 5’-Nucleotidase Superactivity – 447 35.2.7 Thymidine Phosphorylase Deficiency – 447 35.2.8 Thymidine Kinase Deficiency – 447 References – 447 434 Chapter 35 · Disorders of Purine and Pyrimidine Metabolism Purine Metabolism Purine nucleotides are essential cellular constituents 4 The catabolic pathway starts from GMP, IMP and which intervene in energy transfer, metabolic regula- AMP, and produces uric acid, a poorly soluble tion, and synthesis of DNA and RNA. Purine metabo- compound, which tends to crystallize once its lism can be divided into three pathways: plasma concentration surpasses 6.5–7 mg/dl (0.38– 4 The biosynthetic pathway, often termed de novo, 0.47 mmol/l). starts with the formation of phosphoribosyl pyro- 4 The salvage pathway utilizes the purine bases, gua- phosphate (PRPP) and leads to the synthesis of nine, hypoxanthine and adenine, which are pro- inosine monophosphate (IMP). -

Hereditary Orotic Aciduria and Megaloblastic Anaemia: a Second Case, with Response to Uridine
27 February 1965 Specialty Boards in U.S.A.-Buie MMDICAJRNL 547 particular instances by an additional specialist designated by to be completed more quickly; grading is more nearly accurate, the Review Committee. The reports and recommendations of and testing can be more extensive. As a rule the candidate these representatives are forwarded to the Review Committee, becomes eligible for his oral examination after he has passed along with other material prepared by the hospital, and the the " written." The oral examination varies with the require- evaluation by the Committee thus represents the joint action of ments and needs of various specialties. It is important because the Council, the Board, and in some cases the third national it is the last obstacle to be surmounted before the candidate is specialty organization. Depending upon the number of pro- certified. grammes in the various specialties, the related Review Com- Members of specialty boards are conscientious, honest, dedi- mittee meets from once to three times yearly for one or two cated persons who contribute freely of their time and effort, days. All residency programmes are resurveyed normally at often at great sacrifice, to advance the standards of their three-year intervals, but more frequently if the committee so specialty, without thought of personal aggrandizement or recom- directs. pense for their services. Like members of the faculty of a great The standards by which residency training, programmes are university sitting at a defence of a doctoral dissertation, they organized, and against which they are evaluated by the Review are imbued with a sense of their responsibility to determine the Committees, are published by the Council as the Essentials of competence of candidates who appear voluntarily before them Approved Residencies. -

Developmental Disorder Associated with Increased Cellular Nucleotidase Activity (Purine-Pyrimidine Metabolism͞uridine͞brain Diseases)
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 11601–11606, October 1997 Medical Sciences Developmental disorder associated with increased cellular nucleotidase activity (purine-pyrimidine metabolismyuridineybrain diseases) THEODORE PAGE*†,ALICE YU‡,JOHN FONTANESI‡, AND WILLIAM L. NYHAN‡ Departments of *Neurosciences and ‡Pediatrics, University of California at San Diego, La Jolla, CA 92093 Communicated by J. Edwin Seegmiller, University of California at San Diego, La Jolla, CA, August 7, 1997 (received for review June 26, 1997) ABSTRACT Four unrelated patients are described with a represent defects of purine metabolism, although no specific syndrome that included developmental delay, seizures, ataxia, enzyme abnormality has been identified in these cases (6). In recurrent infections, severe language deficit, and an unusual none of these disorders has it been possible to delineate the behavioral phenotype characterized by hyperactivity, short mechanism through which the enzyme deficiency produces the attention span, and poor social interaction. These manifesta- neurological or behavioral abnormalities. Therapeutic strate- tions appeared within the first few years of life. Each patient gies designed to treat the behavioral and neurological abnor- displayed abnormalities on EEG. No unusual metabolites were malities of these disorders by replacing the supposed deficient found in plasma or urine, and metabolic testing was normal metabolites have not been successful in any case. except for persistent hypouricosuria. Investigation of purine This report describes four unrelated patients in whom and pyrimidine metabolism in cultured fibroblasts derived developmental delay, seizures, ataxia, recurrent infections, from these patients showed normal incorporation of purine speech deficit, and an unusual behavioral phenotype were bases into nucleotides but decreased incorporation of uridine. -

Nucleic Acid Metabolism in Regenerating Rat Liver I. the Rate of Deoxyribonucleic Acid Synthesis in Vivo1
Nucleic Acid Metabolism in Regenerating Rat Liver I. The Rate of Deoxyribonucleic Acid Synthesis in Vivo1 LISELOTTEI.HECHTANDVANR. POTTER (McArdie Memorial Laboratory, University of Wisconsin, Madison 6, Wis.) An ideal system for examining the relationship MATERIALS AND METHODS between ribonucleic acid (UNA) and deoxyribo- Partial hepatectomy was performed by the method of nucleic acid (DNA) metabolism in animals would Higgins and Anderson (18) on male albino rats1 weighing 180 be a tissue where synchronous cell division occurs. to 185 gm. The animals were fasted for 16-18 hours before the Isotopie tracer studies (9, 22, 25) and determina operation and fed ad libitum after the operation. At the indi cated times each animal received a single intraperitoneal in tion of the DNA content per nucleus in the liver jection of 1 mg. (5.75 pinoles) of orotic acid-6-C14which con cells of partially hepatectomized rats (25, 26, 83) tained 4.1 X 10* counts/min/mg. Following the injection, have suggested that in animals the most feasible some animals were kept in metabolism cages to facilitate col approach to this situation is by the use of re lection of urine and respiratory CO2. The animals were killed by decapitation. The liver was perfused in situ with ice-cold generating liver. The observations that incorpora 0.25 H sucrose containing 0.00018 M CaCl2, excised, and tion of isotopically labeled precursors into DNA weighed. is correlated with the occurrence of cell division Preparation of cellfractions.'—Theliver was forced through (5,17,30) and that isotopes are retained extensive a plastic mincer, and a 10 per cent homogenate of the liver was ly in the DNA of mitotically inactive and active prepared in 0.25 Msucrose + 0.00018 u CaCl2 (19) with the use cells (2, 4, 6,10-12,16, 31, 32) indicate that DNA of a Potter-Elvehjem glass homogenizer. -

Purine and Pyrimidine Metabolism in Human Epidermis* Jean De Bersaques, Md
THE JOURNAL OP INVESTIGATIVE DERMATOLOGY Vol. 4s, No. Z Copyright 1957 by The Williams & Wilkins Co. Fri nte,1 in U.S.A. PURINE AND PYRIMIDINE METABOLISM IN HUMAN EPIDERMIS* JEAN DE BERSAQUES, MD. The continuous cellular renewal occurring inthine, which contained 5% impurity, and for uric the epidermis requires a very active synthesisacid, which consisted of 3 main components. The reaction was stopped after 1—2 hours in- and breakdown of nuclear and cytoplasmiecubation at 37° and the products were spotted on nucleic acids. Data on the enzyme systemsWhatman 1 filter paper sheets. According to the participating in these metabolic processes arereaction products expected, a choice was made of rather fragmentary (1—9) and some are, inat least 2 among the following solvents, all used terms of biochemical time, in need of up- in ascending direction: 1. isoamyl alcohol—5% Na2HPO4 (1:1), dating. In some other publications (10—18), 2. water-saturated n-butanol, the presence and concentration of various in- 3. distilled water, termediate products is given. 4. 80% formic acid—n-hutanol——n-propanol— In this paper, we tried to collect and supple- acetone—30% trichloro-aeetic acid (5:8:4: ment these data by investigating the presence 5:3), 5. n-butanol——4% boric acid (43:7), or absence in epidermis of enzyme systems 6. isobutyrie acid—water—ammonia 0.880—ver- that have been described in other tissues. sene 0.1M(500:279:21:8), This first investigation was a qualitative one, 7. upper phase of ethyl acetate—water—formic and some limitations were set by practical acid (12:7:1), 8. -

Nitrogen-Stimulated Orotic Acid Synthesis and Nucleotide Imbalance1
[CANCER RESEARCH (SUPPL.) 52. 2082s-2084s. April I. 1992] Nitrogen-stimulated Orotic Acid Synthesis and Nucleotide Imbalance1 Willard J. Visek2 University of Illinois, College of Medicine, Urbana, Illinois 61801 Abstract bound to the inner mitochondria! membrane. The cytoplasmic enzymes reside in two separate multifunctional complexes. One Orotic acid, first discovered in ruminant milk, is an intermediate in contains carbamoyl phosphate synthetase II, aspartate trans- the pyrimidine biosynthesis pathway of animal cells. Its synthesis is carbamylase, and dihydroorotase, whereas the other includes initiated by the formation of carbamoyl phosphate (CP) in the cytoplasm, orotate phosphoribosyl transferase and orotodine-5"-phosphate with ammonia derived from glutamine. Ureotelic species also form CP in the first step of urea synthesis in liver mitochondria. For that, ammonia decarboxylase (2, 3). A deficiency of the latter two enzyme is derived from tissue fluid. When there is insufficient capacity for activities results in accumulation of orotate and a profound rise detoxifying the load of ammonia presented for urea synthesis, CP leaves in its excretion in the urine, a condition known as hereditary the mitochondria and enters the pyrimidine pathway, where orotic acid orotic aciduria (4). This bifunctional protein complex with its biosynthesis is stimulated, orotic acid excretion in urine then increases. two enzyme activities is also referred to as UMP synthase. Orotic acid synthesis is abnormally high with hereditary deficiencies of A severe deficiency of UMP synthase elevates urinary orotic urea-cycle enzymes or uridine monophosphate synthase. It is also ele acid excretion in humans to 1500 mg/day, compared with the vated by ammonia intoxication and during feeding of diets high in protein, usual 2.5 mg/day. -

Metabolite Name Chemical Formula KEGG/HMDB/ Pubchem Identifier Glyoxylate C2H2O3 C00048 Glycolate C2H4O3 C00160 Pyruvate C3H4O3
Metabolite name Chemical formula KEGG/HMDB/ PubChem Identifier glyoxylate C2H2O3 C00048 glycolate C2H4O3 C00160 pyruvate C3H4O3 C00022 lactate C3H6O3 C00186 2-oxobutanoate C4H6O3 C00109 acetoacetate C4H6O3 C00164 glycerate C3H6O4 C00258 uracil C4H4N2O2 C00106 fumarate C4H4O4 C00122 Maleic acid C4H4O4 C01384 2-keto-isovalerate C5H8O3 C00141 Guanidoacetic acid C3H7N3O2 C00581 succinate C4H6O4 C00042 Methylmalonic acid C4H6O4 C02170 3-S-methylthiopropionate C4H8O2S C08276 nicotinate C6H5NO2 C00253 taurine C2H7NO3S C00245 Pyroglutamic acid C5H7NO3 C01879 Citraconic acid C5H6O4 C02226 2-ketohaxanoic acid C6H10O3 HMDB01864 N-Acetyl-L-alanine C5H9NO3 C01073 oxaloacetate C4H4O5 C00036 Hydroxyisocaproic acid C6H12O3 HMDB00746 malate C4H6O5 C00149 hypoxanthine C5H4N4O C00262 anthranilate C7H7NO2 C00108 p-aminobenzoate C7H7NO2 C00568 p-hydroxybenzoate C7H6O3 C00156 acetylphosphate C2H5O5P C00227 Carbamoyl phosphate CH4NO5P C00169 a-ketoglutarate C5H6O5 C00026 Phenylpropiolic acid C9H6O2 HMDB00563 2-oxo-4-methylthiobutanoate C5H8O3S C01180 2-Hydroxy-2-methylbutanedioic acid C5H8O5 C02612 3-methylphenylacetic acid C9H10O2 HMDB02222 xanthine C5H4N4O2 C00385 Hydroxyphenylacetic acid C8H8O3 C05852 2,3-dihydroxybenzoic acid C7H6O4 C00196 orotate C5H4N2O4 C00295 dihydroorotate C5H6N2O4 C00337 allantoin C4H6N4O3 C01551 Aminoadipic acid C6H11NO4 C00956 Indole-3-carboxylic acid C9H7NO2 HMDB03320 phenylpyruvate C9H8O3 C00166 Atrolactic acid C9H10O3 HMDB00475 Phenyllactic acid C9H10O3 C01479 quinolinate C7H5NO4 C03722 phosphoenolpyruvate C3H5O6P C00074 Uric -

Purine and Pyrimidine Metabolism by N. ZOLLNER, Department Of
Proc. Nuti. Soc. (1982), 41,329 329 Purine and pyrimidine metabolism By N. ZOLLNER,Department of Medicine, University of Munchen, West Germany Purines and pyrimidines are essential constituents of animal and plant cells and are contained in various compounds. It is interesting to consider that some of these compounds are very stable, e.g. DNA, while others are rapidly turned over, e.g. ATP. In birds and reptiles uric acid also serves to excrete nitrogen. The aim of this paper is to give a short review of purine and pyrimidine metabolism and to describe in some detail aspects important to the field of nutrition, with emphasis placed on work done in vitro and in man. Purines The most important structure in purine biochemistry is the nucleotide consisting of a purine base, ribose or deoxyribose, and phosphoric acid. The most important purine bases are adenine, guanine, hypoxanthine and xanthine. The ribosides of all of them are known to occur in metabolism. Adenine and guanine themselves are usually not found in the tissues of mammals, but free hypoxanthine and xanthine are intermediates in the degradation of purines. Uric acid is a divalent acid, but the second dissociation constant is so small that at around pH 7 only the monobasic salts are formed. These are sparingly soluble in the body fluids. Peters & van Slyke (1946) have calculated a maximum solubility of 6.5 mg/Ioo ml (as uric acid) in plasma. Gout and uric acid nephropathies are due to this low solubility. Nucleosides are pentose-glycosides containing ribose or deoxyribose. Normally the linkage is with atom 9, but nucleosides with atom 3 do occur. -

Diagnose a Broad Range of Metabolic Disorders with a Single Test, Global
PEDIATRIC Assessing or diagnosing a metabolic disorder commonly requires several tests. Global Metabolomic Assisted Pathway Screen, commonly known as Global MAPS, is a unifying test GLOBAL MAPS™ for analyzing hundreds of metabolites to identify changes Global Metabolomic or irregularities in biochemical pathways. Let Global MAPS Assisted Pathway Screen guide you to an answer. Diagnose a broad range of metabolic disorders with a single test, Global MAPS Global MAPS is a large scale, semi-quantitative metabolomic profiling screen that analyzes disruptions in both individual analytes and pathways related to biochemical abnormalities. Using state-of-the-art technologies, Global Metabolomic Assisted Pathway Screen (Global MAPS) provides small molecule metabolic profiling to identify >700 metabolites in human plasma, urine, or cerebrospinal fluid. Global MAPS identifies inborn errors of metabolism (IEMs) that would ordinarily require many different tests. This test defines biochemical pathway errors not currently detected by routine clinical or genetic testing. IEMs are inherited metabolic disorders that prevent the body from converting one chemical compound to another or from transporting a compound in or out of a cell. NORMAL PROCESS METABOLIC ERROR These processes are necessary for essentially all bodily functions. Most IEMs are caused by defects in the enzymes that help process nutrients, which result in an accumulation of toxic substances or a deficiency of substances needed for normal body function. Making a swift, accurate diagnosis -

The Molecular Basis and Pathology of Phenotypic Variability in Urea Cycle Disorders
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2014 The Molecular Basis and Pathology of Phenotypic Variability in Urea Cycle Disorders Hu, Lyian Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-108004 Dissertation Published Version Originally published at: Hu, Lyian. The Molecular Basis and Pathology of Phenotypic Variability in Urea Cycle Disorders. 2014, University of Zurich, Faculty of Science. The Molecular Basis and Pathology of Phenotypic Variability in Urea Cycle Disorders Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Liyan HU aus Taizhou, Zhejiang der V.R. China Promotionskomitee Prof. Dr. sc. nat. Beat W. Schäfer (Vorsitz) Prof. Dr. med. Johannes Häberle (Leitung der Dissertation) Prof. Dr. sc. nat. Thierry Hennet PD Dr. med. Jean-Marc Nuoffer Zürich, March 2014 The present study was performed from October 2010 till March 2014 in the metabolic laboratory at the Division of Metabolism, University Children’s Hospital Zürich under the supervision of Prof. Dr. med. Johannes Häberle. Publications represented in this study: 1. Understanding the Role of Argininosuccinate Lyase Transcript Variants in the Clinical and Biochemical Variability of the Urea Cycle Disorder Argininosuccinic Aciduria Liyan Hu, Amit V. Pandey, Sandra Eggimann, Véronique Rüfenacht, Dorothea Möslinger, Jean-Marc Nuoffer, Johannes Häberle (2013) The Journal of biological chemistry 288(48), 34599-34611 2. Variant forms of the urea cycle disorder argininosuccinic aciduria are caused by folding defects of argininosuccinate lyase Liyan Hu, Amit V. -
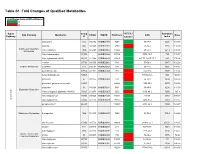
Table S1. Fold Changes of Qualified Metabolites
Table S1. Fold Changes of Qualified Metabolites Fold Change: Cases of WTC-LI/Control <1 >1 Super Comp WTC-LI Retention Sub Pathway Metabolite KEGG HMDB PubChem CAS Mass Pathway ID Control Index asparagine 512 C00152 HMDB00168 6267 0.99 70-47-3 1225 133.06 aspartate 443 C00049 HMDB00191 5960 1.09 56-84-8 1165 134.04 Alanine and Aspartate N-acetylalanine 1585 C02847 HMDB00766 88064 0.99 97-69-8 861.2 130.05 Metabolism N-acetylasparagine 33942 HMDB06028 99715 0.82 4033-40-3 785 175.07 N-acetylaspartate (NAA) 22185 C01042 HMDB00812 65065 0.95 997-55-7;997-55-7 880 176.06 creatine 27718 C00300 HMDB00064 586 0.95 57-00-1 1947 132.08 Creatine Metabolism creatinine 513 C00791 HMDB00562 588 0.99 60-27-5 2055 114.07 guanidinoacetate 43802 C00581 HMDB00128 763 1.05 352-97-6 1937 118.06 beta-citrylglutamate 54923 1.05 73590-26-8 900 322.08 glutamate 57 C00025 HMDB00148 611 1.06 56-86-0 1500 148.06 glutamate, gamma-methyl ester 33487 68662 0.89 1499-55-4 2170 162.08 glutamine 53 C00064 HMDB00641 5961 0.89 56-85-9 1291 147.08 Glutamate Metabolism N-acetyl-aspartyl-glutamate (NAAG) 35665 C12270 HMDB01067 5255 1.04 3106-85-2 1035 305.1 N-acetylglutamate 15720 C00624 HMDB01138 70914 0.84 8/3/17 1050 190.07 Amino Acid Amino N-acetylglutamine 33943 C02716 HMDB06029 182230 0.48 2490-97-3 2140 187.07 pyroglutamine* 46225 134508 0.89 2353-44-8 1900 129.07 Glutathione Metabolism 5-oxoproline 1494 C01879 HMDB00267 7405 0.93 98-79-3 738.5 128.04 allo-threonine 15142 C05519 HMDB04041 99289 0.91 28954-12-3 2511.1 118.05 betaine 3141 C00719 HMDB00043 247 0.92 107-43-7 -

Defects in Metabolism of Purines and Pyrimidines
Ned Tijdschr Klin Chem 1999; 24: 171-175 Defects in metabolism of purines and pyrimidines A.H. van GENNIP Defects in the metabolism of purines and pyrimidines To date 27 defects of purine and pyrimidine metabo- are not well-known in the general hospital. For this lism have been documented. They are listed in Tables reason relatively few patients suffering from these 1 and 2. diseases are being diagnosed. However, at present 27 different defects of purine- and pyrimidine metabo- Diagnosis lism have already been documented. Clinically, these In purine metabolism uric acid is the end product of defects are not easily recognised, at least for the larger biosynthesis 'de novo', salvage and degradation and part, because of non-specific symptoms. Therefore, therefore measurement of uric acid in plasma and the assistance of a clinical chemistry laboratory spe- urine will lead to an indication for several purine cialized in inborn errors is indispensable to discover defects but certainly not all defects (table 1). Pyrim- most of these defects. This review describes the various idine metabolism does not have such an end product. biochemical and clinical aspects of the defects of purine Moreover, as in many inborn errors of metabolism and pyrimidine metabolism and provides a guide for clinical symptomatology is aspecific and highly variable their detection, diagnosis and treatment. (Table 3). Therefore, screening methods covering a broad spectrum of purine and pyrimidine metabolites Definition and frequency will provide the best possibility of detecting most of Defects of purine and pyrimidine metabolism are the known defects or even new defects. Such methods characterized by abnormal concentrations of purines, are already operative in many centres around the pyrimidines and/or their metabolites in cells or body world for amino acids, organic acids, mucopolys- fluids due to a decreased or an increased activity of accharides and oligosaccharides.