Drugs Inducing Hearing Loss, Tinnitus, Dizziness and Vertigo: an Updated Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
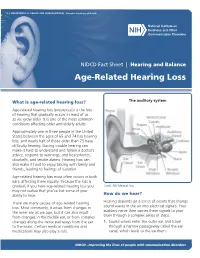
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -
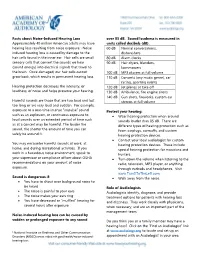
Facts About Noise-Induced Hearing Loss Over 85 Db
Facts about Noise-Induced Hearing Loss over 85 dB. Sound loudness is measured in Approximately 40 million American adults may have units called decibels (dB). hearing loss resulting from noise exposure.1 Noise- 60 dB Normal conversations, induced hearing loss is caused by damage to the dishwashers hair cells found in the inner ear. Hair cells are small 80 dB Alarm clocks sensory cells that convert the sounds we hear 90 dB Hair dryers, blenders, (sound energy) into electrical signals that travel to lawnmowers the brain. Once damaged, our hair cells cannot 100 dB MP3 players at full volume grow back, which results in permanent hearing loss. 110 dB Concerts (any music genre), car racing, sporting events Hearing protection decreases the intensity, or 120 dB Jet planes at take off loudness, of noise and helps preserve your hearing. 130 dB Ambulance, fire engine sirens 140 dB Gun shots, fireworks, custom car Harmful sounds are those that are too loud and last stereos at full volume too long or are very loud and sudden. For example, exposure to a one-time intense “impulse” sound Protect your hearing: such as an explosion, or continuous exposure to • Wear hearing protection when around loud sounds over an extended period of time such sounds louder than 85 dB. There are as at a concert may be harmful. The louder the different types of hearing protection such as sound, the shorter the amount of time you can foam earplugs, earmuffs, and custom safely be around it. hearing protection devices • Contact your local audiologist for custom You may encounter harmful sounds at work, at hearing protection devices. -

Migraine Associated Vertigo
Headache: The Journal of Head and Face Pain VC 2015 American Headache Society Published by JohnWiley & Sons, Inc. doi: 10.1111/head.12704 Headache Toolbox Migraine Associated Vertigo Between 30 and 50% of migraineurs will sometimes times a condition similar to benign positional vertigo experience dizziness, a sense of spinning, or feeling like called vestibular neuronitis (or vestibular neuritis/labyrinthi- their balance is off in the midst of their headaches. This is tis) is triggered by a viral infection of the inner ear, result- now termed vestibular migraine, but is also called ing in constant vertigo or unsteadiness. Symptoms can migraine associated vertigo. Sometimes migraineurs last for a few days to a few weeks and then go away as experience these symptoms before the headache, but mysteriously as they came on. Vestibular migraine, by they can occur during the headache, or even without any definition, should have migraine symptoms in at least head pain. In children, vertigo may be a precursor to 50% of the vertigo episodes, and these include head migraines developing in the teens or adulthood. Migraine pain, light and noise sensitivity, and nausea. associated vertigo may be more common in those with There are red flags, which are warning signs that ver- motion sickness. tigo is not part of a migraine. Sudden hearing loss can be For some patients this vertiginous sensation resem- the sign of an infection that needs treatment. Loss of bal- bles migraine aura, which is a reversible, relatively short- ance alone, or accompanied by weakness can be the lived neurologic symptom associated with their migraines. -

Objective Measures of Ototoxicity
The following article appeared in the September 2005 issue (Vol. 9, No. 1, pp. 10-16) of the Division 6 publication Perspectives on Hearing and Hearing Disorders: Research and Diagnostics. To learn more about Division 6, contact the ASHA Action Center at 1-800-498-2071 or visit the division’s Web page at www.asha.org/about/membership-certification/divs/div_6.htm. Objective Measures of Ototoxicity Elizabeth Leigh-Paffenroth Veterans Affairs Rehabilitation Research and Development Service, National Center for Rehabilitative Auditory Research, VA Medical Center Portland, OR Department of Otolaryngology, Oregon Health & Science University Portland, OR Kelly M. Reavis, Jane S. Gordon, Kathleen T. Dunckley Veterans Affairs Rehabilitation Research and Development Service, National Center for Rehabilitative Auditory Research, VA Medical Center Portland, OR Stephen A. Fausti and Dawn Konrad-Martin Veterans Affairs Rehabilitation Research and Development Service, National Center for Rehabilitative Auditory Research, VA Medical Center Portland, OR Department of Otolaryngology, Oregon Health & Science University, Portland, OR A leading cause of preventable sensorineural hear- of patients who are unable to provide reliable re- ing loss is therapeutic treatment with medications that sponses; subsequently, many of these patients do not are toxic to inner ear tissues, including certain drugs receive monitoring for ototoxic-induced changes in used to fight cancer and life-threatening infectious dis- their hearing. The development of objective measures eases. Ototoxic-induced hearing loss typically begins that do not require patient cooperation is necessary to in the high frequencies and progresses to lower fre- monitor all patients receiving ototoxic drugs. quencies as drug administration continues (Campbell Two objective measures offer promise in their abil- & Durrant, 1993; Campbell et al., 2003; Macdonald, ity to detect and to monitor hearing changes caused by Harrison, Wake, Bliss, & Macdonald, 1994). -

Vestibular Neuritis and Labyrinthitis
Vestibular Neuritis and DISORDERS Labyrinthitis: Infections of the Inner Ear By Charlotte L. Shupert, PhD with contributions from Bridget Kulick, PT and the Vestibular Disorders Association INFECTIONS Result in damage to inner ear and/or nerve. ARTICLE 079 DID THIS ARTICLE HELP YOU? SUPPORT VEDA @ VESTIBULAR.ORG Vestibular neuritis and labyrinthitis are disorders resulting from an 5018 NE 15th Ave. infection that inflames the inner ear or the nerves connecting the inner Portland, OR 97211 ear to the brain. This inflammation disrupts the transmission of sensory 1-800-837-8428 information from the ear to the brain. Vertigo, dizziness, and difficulties [email protected] with balance, vision, or hearing may result. vestibular.org Infections of the inner ear are usually viral; less commonly, the cause is bacterial. Such inner ear infections are not the same as middle ear infections, which are the type of bacterial infections common in childhood affecting the area around the eardrum. VESTIBULAR.ORG :: 079 / DISORDERS 1 INNER EAR STRUCTURE AND FUNCTION The inner ear consists of a system of fluid-filled DEFINITIONS tubes and sacs called the labyrinth. The labyrinth serves two functions: hearing and balance. Neuritis Inflamation of the nerve. The hearing function involves the cochlea, a snail- shaped tube filled with fluid and sensitive nerve Labyrinthitis Inflamation of the labyrinth. endings that transmit sound signals to the brain. Bacterial infection where The balance function involves the vestibular bacteria infect the middle organs. Fluid and hair cells in the three loop-shaped ear or the bone surrounding semicircular canals and the sac-shaped utricle and Serous the inner ear produce toxins saccule provide the brain with information about Labyrinthitis that invade the inner ear via head movement. -

Vestibular Neuritis, Labyrinthitis, and a Few Comments Regarding Sudden Sensorineural Hearing Loss Marcello Cherchi
Vestibular neuritis, labyrinthitis, and a few comments regarding sudden sensorineural hearing loss Marcello Cherchi §1: What are these diseases, how are they related, and what is their cause? §1.1: What is vestibular neuritis? Vestibular neuritis, also called vestibular neuronitis, was originally described by Margaret Ruth Dix and Charles Skinner Hallpike in 1952 (Dix and Hallpike 1952). It is currently suspected to be an inflammatory-mediated insult (damage) to the balance-related nerve (vestibular nerve) between the ear and the brain that manifests with abrupt-onset, severe dizziness that lasts days to weeks, and occasionally recurs. Although vestibular neuritis is usually regarded as a process affecting the vestibular nerve itself, damage restricted to the vestibule (balance components of the inner ear) would manifest clinically in a similar way, and might be termed “vestibulitis,” although that term is seldom applied (Izraeli, Rachmel et al. 1989). Thus, distinguishing between “vestibular neuritis” (inflammation of the vestibular nerve) and “vestibulitis” (inflammation of the balance-related components of the inner ear) would be difficult. §1.2: What is labyrinthitis? Labyrinthitis is currently suspected to be due to an inflammatory-mediated insult (damage) to both the “hearing component” (the cochlea) and the “balance component” (the semicircular canals and otolith organs) of the inner ear (labyrinth) itself. Labyrinthitis is sometimes also termed “vertigo with sudden hearing loss” (Pogson, Taylor et al. 2016, Kim, Choi et al. 2018) – and we will discuss sudden hearing loss further in a moment. Labyrinthitis usually manifests with severe dizziness (similar to vestibular neuritis) accompanied by ear symptoms on one side (typically hearing loss and tinnitus). -

ICD-9 Diseases of the Ear and Mastoid Process 380-389
DISEASES OF THE EAR AND MASTOID PROCESS (380-389) 380 Disorders of external ear 380.0 Perichondritis of pinna Perichondritis of auricle 380.00 Perichondritis of pinna, unspecified 380.01 Acute perichondritis of pinna 380.02 Chronic perichondritis of pinna 380.1 Infective otitis externa 380.10 Infective otitis externa, unspecified Otitis externa (acute): NOS circumscribed diffuse hemorrhagica infective NOS 380.11 Acute infection of pinna Excludes: furuncular otitis externa (680.0) 380.12 Acute swimmers' ear Beach ear Tank ear 380.13 Other acute infections of external ear Code first underlying disease, as: erysipelas (035) impetigo (684) seborrheic dermatitis (690.10-690.18) Excludes: herpes simplex (054.73) herpes zoster (053.71) 380.14 Malignant otitis externa 380.15 Chronic mycotic otitis externa Code first underlying disease, as: aspergillosis (117.3) otomycosis NOS (111.9) Excludes: candidal otitis externa (112.82) 380.16 Other chronic infective otitis externa Chronic infective otitis externa NOS 380.2 Other otitis externa 380.21 Cholesteatoma of external ear Keratosis obturans of external ear (canal) Excludes: cholesteatoma NOS (385.30-385.35) postmastoidectomy (383.32) 380.22 Other acute otitis externa Excerpted from “Dtab04.RTF” downloaded from website regarding ICD-9-CM 1 of 11 Acute otitis externa: actinic chemical contact eczematoid reactive 380.23 Other chronic otitis externa Chronic otitis externa NOS 380.3 Noninfectious disorders of pinna 380.30 Disorder of pinna, unspecified 380.31 Hematoma of auricle or pinna 380.32 Acquired -

Hearing Loss, Vertigo and Tinnitus
HEARING LOSS, VERTIGO AND TINNITUS Jonathan Lara, DO April 29, 2012 Hearing Loss Facts S Men are more likely to experience hearing loss than women. S Approximately 17 percent (36 million) of American adults report some degree of hearing loss. S About 2 to 3 out of every 1,000 children in the United States are born deaf or hard-of-hearing. S Nine out of every 10 children who are born deaf are born to parents who can hear. Hearing Loss Facts S The NIDCD estimates that approximately 15 percent (26 million) of Americans between the ages of 20 and 69 have high frequency hearing loss due to exposure to loud sounds or noise at work or in leisure activities. S Only 1 out of 5 people who could benefit from a hearing aid actually wears one. S Three out of 4 children experience ear infection (otitis media) by the time they are 3 years old. Hearing Loss Facts S There is a strong relationship between age and reported hearing loss: 18 percent of American adults 45-64 years old, 30 percent of adults 65-74 years old, and 47 percent of adults 75 years old or older have a hearing impairment. S Roughly 25 million Americans have experienced tinnitus. S Approximately 4,000 new cases of sudden deafness occur each year in the United States. Hearing Loss Facts S Approximately 615,000 individuals have been diagnosed with Ménière's disease in the United States. Another 45,500 are newly diagnosed each year. S One out of every 100,000 individuals per year develops an acoustic neurinoma (vestibular schwannoma). -

Hearing Loss
Randal W. Swenson, M.D. Joshua G. Yorgason, M.D. David K. Palmer, M.D. Wesley R. Brown, M.D. John E. Butler, M.D. Nancy J. Stevenson, PA-C Justin D. Gull, M.D. ENT SPECIALISTS Kristin G. Hoopes, PA-C www.entslc.com Hearing Loss Approximately one in ten persons in the United may result from blockage of the ear canal (wax), States has some degree of hearing loss. Hearing is from a perforation (hole) in the ear drum, or from measured in decibels (dB), and a hearing level of 0- infection or disease of any of the three middle ear 25 dB is considered normal hearing. Your level is: bones. With a conductive loss only, the patient will never go deaf, but will always be able to hear, either Right ear _______ dB Left ear _______dB with reconstructive ear surgery or by use of a properly fitted hearing aid. Some patients who are Hearing Severity / % Loss not candidates for surgery, may benefit from a new 25 dB (normal).….0% 65dB(Severe)……...60% technology, the Baha (bone-anchored hearing aid). 35 dB (mild)……..15% 75dB(Severe)……...75% When there is a problem with the inner ear or 45 dB (moderate)..30% >85dB (Profound)..>90% nerve of hearing, a sensori-neural hearing loss occurs. This is most commonly from normal aging, Normal speech discrimination is 88-100%. Yours is: is usually worse in high frequencies, and can progress to total deafness. Noise exposure is another Right ear _______ % Left ear_______% common cause of high frequency hearing loss. Patients with sensori-neural hearing loss usually complain of difficulty hearing in loud environments. -

Sports Injuries
Sports Injuries Of the Ear G.A.WAGNER,MD SUMMARY The author describes common sports injuries involving the ear. Such injuries include hematoma, lacerations, foreign bodies (tattoo), and thermal injuries. Ear canal injuries include swimmer's ear and penetrating injuries. Tympanum injuries include tympanic membrane perforations, ossicular discontinuity, eustachian tube dysfunction, temporal bone fractures and traumatic facial nerve palsy. Inner ear injuries include traumatic sensorineural deafness. The author emphasizes the management of these injuries. Dr. Wagner, an otolaryngologist, is on the active staff of three Calgary hospitals. SPORTS INJURIES to the ear are as variable as the frostbite. These are usually found in skiers, skaters, and activities which cause them - they may involve the mountain climbers. Severe, deep frostbite injuries of the auricle, external auditory canal, tympanum, and inner ear, auricle are best treated by rapid rewarming with water- but only rarely involve the eighth cranial nerve or central soaked pledgets maintained at between 38 and 42 degrees auditory pathways. centigrade. Freezing, thawing, and re-freezing must be avoided since this is extremely injurious to tissue.2 Surgical Auricle debridement may occasionally be required but is generally Auricle injuries are common, as there is a human reflex prohibited for several months as the auricle has an amazing tendency to turn the head to the side when expecting capacity for self repair. frontal head trauma. The auricle is thereby involved even though the impact may have been from a head-on External Auditory Canal direction.' Lacerations in the ear canal, if minor, may be treated The so-called 'cauliflower ear' often seen in retired with an antibiotic-steroid eardrop solution in order to boxers or wrestlers is caused by untreated - or repeated - minimize crusting, maximize clot dissolution and cleansing hematomas of the auricle. -

Benign Paroxysmal Positional Vertigo (BPPV)
Patient Information Patient Information Benign Paroxysmal Positional Vertigo (BPPV) BPPV causes spinning dizziness (‘vertigo’) due to crystals in the inner ear breaking loose. The crystals are normally embedded in a jelly and when we move forwards and backwards, or up and down, in space they send our brain messages about where we are. If they become loose they can find their way into the wrong area and cause the sensation that we are moving when we are actually not – the result is a sudden feeling of spinning, usually set off by turning your head. What causes BPPV? Perhaps half of all cases of BPPV are "idiopathic" – they occur for no known reason. The most common identified cause of BPPV in people under fifty is a previous head injury. In older people the most common cause is ‘wear and tear’ of the vestibular (balance) system of the inner ear. Some people have a history of previous inner ear inflammation with a period of severe, prolonged dizziness. Diagnosing BPPV The diagnosis is made based on history, physical examination and sometimes with hearing or balance testing. Other diagnostic tests may be required: for example, a CT or MRI may be required for cases that don't fit the usual pattern. It is possible to have BPPV in both ears, which may make the diagnosis and treatment more challenging. How is BPPV Treated? BPPV is often described as ‘self-limiting’ because symptoms often subside or disappear without any medical treatment. Symptoms tend to wax and wane. Physical manoeuvres and exercises are very effective in stopping your symptoms, and are the main basis of treatment. -

Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure
Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure Safety and Health Information Bulletin SHIB 03-08-2018 DHHS (NIOSH) Publication No. 2018-124 Introduction Millions of workers are exposed to noise in the workplace every day and when uncontrolled, noise exposure may cause permanent hearing loss. Research demonstrates exposure to certain chemicals, called ototoxicants, may cause hearing loss or balance problems, regardless of noise exposure. Substances including certain pesticides, solvents, and pharmaceuticals that contain ototoxicants can negatively affect how the ear functions, causing hearing loss, and/or affect balance. Source/Copyright: OSHA The risk of hearing loss is increased when workers are exposed to these chemicals while working around elevated noise levels. This combination often results in hearing loss that can be temporary or permanent, depending on the level of noise, the dose of the chemical, and the duration of the exposure. This hearing impairment affects many occupations and industries, from machinists to firefighters. Effects on Hearing Harmful exposure to ototoxicants may occur through inhalation, ingestion, or skin absorption. Health effects caused by ototoxic chemicals vary based on exposure frequency, intensity, duration, workplace exposure to other hazards, and individual factors such as age. Effects may be temporary or permanent, can affect hearing sensitivity and result in a standard threshold shift. Since chemicals can affect central portions of the auditory system (e.g., nerves or nuclei in the central nervous system, the pathways to the brain or in the brain itself), not only do sounds need to be louder to be detected, but also they lose clarity. Specifically, speech discrimination dysfunction, the ability to hear voices separately from background noise, may occur and involve: .