A Phylogenetic Perspective on the Evolution of Mediterranean Teleost Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mediterranean Marine Science
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by National Documentation Centre - EKT journals Mediterranean Marine Science Vol. 20, 2019 Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters KOVAČIĆ MARCELO Natural History Museum Rijeka SCHEMBRI PATRICK University of Malta https://doi.org/10.12681/mms.19816 Copyright © 2019 Mediterranean Marine Science To cite this article: KOVAČIĆ, M., & SCHEMBRI, P. (2019). Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters. Mediterranean Marine Science, 20(2), 287-296. doi:https://doi.org/10.12681/mms.19816 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 23/03/2020 08:31:30 | Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.19816 Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters Marcelo KOVAČIĆ¹ and Patrick J. SCHEMBRI² ¹Natural History Museum Rijeka, Lorenzov prolaz 1, HR-51000 Rijeka ²Department of Biology, University of Malta, Msida MSD2080, Malta Corresponding author: [email protected] Handling Editor: Argyro ZENETOS Received: 25 February 2019; Accepted: 23 March 2019; Published on line: 28 May 2019 Abstract Twelve new first records of species from two families are added to the list of known marine fishes from Malta based on labo- ratory study of material collected during fieldwork over a period of more than twenty years. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Order GASTEROSTEIFORMES PEGASIDAE Eurypegasus Draconis
click for previous page 2262 Bony Fishes Order GASTEROSTEIFORMES PEGASIDAE Seamoths (seadragons) by T.W. Pietsch and W.A. Palsson iagnostic characters: Small fishes (to 18 cm total length); body depressed, completely encased in Dfused dermal plates; tail encircled by 8 to 14 laterally articulating, or fused, bony rings. Nasal bones elongate, fused, forming a rostrum; mouth inferior. Gill opening restricted to a small hole on dorsolat- eral surface behind head. Spinous dorsal fin absent; soft dorsal and anal fins each with 5 rays, placed posteriorly on body. Caudal fin with 8 unbranched rays. Pectoral fins large, wing-like, inserted horizon- tally, composed of 9 to 19 unbranched, soft or spinous-soft rays; pectoral-fin rays interconnected by broad, transparent membranes. Pelvic fins thoracic, tentacle-like,withI spine and 2 or 3 unbranched soft rays. Colour: in life highly variable, apparently capable of rapid colour change to match substrata; head and body light to dark brown, olive-brown, reddish brown, or almost black, with dorsal and lateral surfaces usually darker than ventral surface; dorsal and lateral body surface often with fine, dark brown reticulations or mottled lines, sometimes with irregular white or yellow blotches; tail rings often encircled with dark brown bands; pectoral fins with broad white outer margin and small brown spots forming irregular, longitudinal bands; unpaired fins with small brown spots in irregular rows. dorsal view lateral view Habitat, biology, and fisheries: Benthic, found on sand, gravel, shell-rubble, or muddy bottoms. Collected incidentally by seine, trawl, dredge, or shrimp nets; postlarvae have been taken at surface lights at night. -

Mediterranean Marine Science
Mediterranean Marine Science Vol. 20, 2019 Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters KOVAČIĆ MARCELO Natural History Museum Rijeka SCHEMBRI PATRICK University of Malta http://dx.doi.org/10.12681/mms.19816 Copyright © 2019 Mediterranean Marine Science To cite this article: KOVAČIĆ, M., & SCHEMBRI, P. (2019). Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters. Mediterranean Marine Science, 20(2), 287-296. doi:http://dx.doi.org/10.12681/mms.19816 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 09/09/2019 20:56:43 | Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.19816 Twelve new records of gobies and clingfishes (Pisces: Teleostei) significantly increase small benthic fish diversity of Maltese waters Marcelo KOVAČIĆ¹ and Patrick J. SCHEMBRI² ¹Natural History Museum Rijeka, Lorenzov prolaz 1, HR-51000 Rijeka ²Department of Biology, University of Malta, Msida MSD2080, Malta Corresponding author: [email protected] Handling Editor: Argyro ZENETOS Received: 25 February 2019; Accepted: 23 March 2019; Published on line: 28 May 2019 Abstract Twelve new first records of species from two families are added to the list of known marine fishes from Malta based on labo- ratory study of material collected during fieldwork over a period of more than twenty years. The three new records of clingfishes (Apletodon incognitus, Gouania wildenowi, Opeatogenys gracilis) double the known diversity of Gobiesocidae for the area; the nine new records of gobies (Chromogobius zebratus, Gobius ater, Gobius fallax, Gobius gasteveni, Gobius roulei, Lebetus guilleti, Odondebuenia balearica, Speleogobius llorisi, Vanneaugobius dollfusi) increase by almost a half the known diversity of Maltese Gobiidae. -

Otolith Atlas for the Western Mediterranean, North and Central Eastern Atlantic
SCIENTIA MARINA 72S1 July 2008, 7-198, Barcelona (Spain) ISSN: 0214-8358 Otolith atlas for the western Mediterranean, north and central eastern Atlantic VICTOR M. TUSET 1, ANTONI LOMBARTE 2 and CARLOS A. ASSIS 3 1 Instituto Canario de Ciencias Marinas, Departamento de Biología Pesquera, P.O. Box. 56, E-35200 Telde (Las Palmas), Canary Islands, Spain. E-mail: [email protected] 2 Institut de Ciències del Mar-CSIC, Departament de Recursos Marins Renovables, Passeig Marítim 37-49, Barcelona 08003, Catalonia, Spain. 3 Instituto de Oceanografia e Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, Campo Grande 1749-016, Lisboa, Portugal. SUMMARY: The sagittal otolith of 348 species, belonging to 99 families and 22 orders of marine Teleostean fishes from the north and central eastern Atlantic and western Mediterranean were described using morphological and morphometric characters. The morphological descriptions were based on the otolith shape, outline and sulcus acusticus features. The mor- phometric parameters determined were otolith length (OL, mm), height (OH, mm), perimeter (P; mm) and area (A; mm2) and were expressed in terms of shape indices as circularity (P2/A), rectangularity (A/(OL×OH)), aspect ratio (OH/OL; %) and OL/fish size. The present Atlas provides information that complements the characterization of some ichthyologic taxa. In addition, it constitutes an important instrument for species identification using sagittal otoliths collected in fossiliferous layers, in archaeological sites or in feeding remains of bony fish predators. Keywords: otolith, sagitta, morphology, morphometry, western Mediterranean, north eastern Atlantic, central eastern Atlantic. RESUMEN: Otolitos de peces del mediterráneo occidental y del atlántico central y nororiental. -

FAMILY Ophichthidae Gunther, 1870
FAMILY Ophichthidae Gunther, 1870 - snake eels and worm eels SUBFAMILY Myrophinae Kaup, 1856 - worm eels [=Neenchelidae, Aoteaidae, Muraenichthyidae, Benthenchelyini] Notes: Myrophinae Kaup, 1856a:53 [ref. 2572] (subfamily) Myrophis [also Kaup 1856b:29 [ref. 2573]] Neenchelidae Bamber, 1915:478 [ref. 172] (family) Neenchelys [corrected to Neenchelyidae by Jordan 1923a:133 [ref. 2421], confirmed by Fowler 1934b:163 [ref. 32669], by Myers & Storey 1956:21 [ref. 32831] and by Greenwood, Rosen, Weitzman & Myers 1966:393 [ref. 26856]] Aoteaidae Phillipps, 1926:533 [ref. 6447] (family) Aotea [Gosline 1971:124 [ref. 26857] used Aotidae; family name sometimes seen as Aoteidae or Aoteridae] Muraenichthyidae Whitley, 1955b:110 [ref. 4722] (family) Muraenichthys [name only, used as valid before 2000?; not available] Benthenchelyini McCosker, 1977:13, 57 [ref. 6836] (tribe) Benthenchelys GENUS Ahlia Jordan & Davis, 1891 - worm eels [=Ahlia Jordan [D. S.] & Davis [B. M.], 1891:639] Notes: [ref. 2437]. Fem. Myrophis egmontis Jordan, 1884. Type by original designation (also monotypic). •Valid as Ahlia Jordan & Davis, 1891 -- (McCosker et al. 1989:272 [ref. 13288], McCosker 2003:732 [ref. 26993], McCosker et al. 2012:1191 [ref. 32371]). Current status: Valid as Ahlia Jordan & Davis, 1891. Ophichthidae: Myrophinae. Species Ahlia egmontis (Jordan, 1884) - key worm eel [=Myrophis egmontis Jordan [D. S.], 1884:44, Leptocephalus crenatus Strömman [P. H.], 1896:32, Pl. 3 (figs. 4-5), Leptocephalus hexastigma Regan [C. T.] 1916:141, Pl. 7 (fig. 6), Leptocephalus humilis Strömman [P. H.], 1896:29, Pl. 2 (figs. 7-9), Myrophis macrophthalmus Parr [A. E.], 1930:10, Fig. 1 (bottom), Myrophis microps Parr [A. E.], 1930:11, Fig. 1 (top)] Notes: [Proceedings of the Academy of Natural Sciences of Philadelphia v. -

Contents of Volume 42 (2012)
CONTENTS OF VOLUME 42 (2012) Issue 1 Tripp-Valdez A., Arreguín-Sánchez F., Zetina-Rejón M.J. The food of Selene peruviana (Actinopterygii: Perciformes: Carangidae) in the southern Gulf of California .................. 1 Zarrad R., Alemany F., Jarboui O., Garcia A., Akrout F. Comparative characterization of the spawning environments of European anchovy, Engraulis encrasicolus , and round sardinella, Sardinella aurita (Actinopterygii: Clupeiformes) in the eastern coast of Tunisia ............................... 9 Ordines F., Valls M., Gouraguine A. Biology, feeding, and habitat preferences of Cadenat’s rockfish, Scorpaena loppei (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Balearic Islands (western Mediterranean) ..................................... 21 Socha M., Sokołowska-Mikołajczyk M., Szczerbik P., Chyb J., Mikołajczyk T., Epler P. The effect of polychlorinated biphenyls mixture (Aroclor 1254) on the embryonic development and hatching of Prussian carp, Carassius gibelio , and common carp, Cyprinus carpio (Actinopterygii: Cypriniformes: Cyprinidae) ............................................................................................................................................................... 31 Khan A., Ghosh K. Characterization and identification of gut-associated phytase-producing bacteria in some fresh water fish cultured in ponds ...................................................................................................................................................................................... -

(Teleostei: Gobiidae) from the Canary Islands
Zootaxa 3793 (4): 453–464 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3793.4.4 http://zoobank.org/urn:lsid:zoobank.org:pub:847946D4-6AB2-4B36-8BB1-0CA59E60511D A new species of Didogobius (Teleostei: Gobiidae) from the Canary Islands JAMES L. VAN TASSELL1 & ANNEMARIE KRAMER2 1Department of Ichthyology, American Museum of Natural History, New York, NY 10024. E-mail: [email protected] 2School for Field Studies, Bocas del Toro, Panama. E-mail: [email protected] Abstract Didogobius helenae is described from the Canary Islands. It has a sensory papillae pattern that is consistent with the cur- rent diagnosis for Didogobius, but lacks all head canals and pores that are present in other members of the genus. Pores, in general, are replaced by large papillae. The species is defined by first dorsal fin VI; second dorsal fin I,10; anal fin I,9; pectoral fin 16–17; pelvic fin I,5 and disk shaped; lateral scales 28–30, cycloid at anterior, becoming ctenoid posteriorly; cycloid scales present on belly and posterior breast; predorsal region, cheek, operculum and base of pectoral fin without scales; lower most scale on the caudal fin-base with elongate, thickened ctenii along the upper and lower posterior edges. Color in life consists of four mottled, wide brown-orange bars separated by narrower white bars on the trunk, the cheek whitish with 5 more or less circular blotches of orange, outlined in dark brown and a black spot on ventral operculum. -
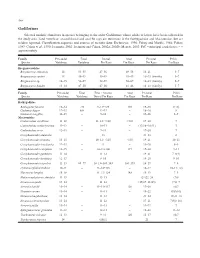
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Trade in Seahorses and Other Syngnathids in Countries Outside Asia (1998-2001)
ISSN 1198-6727 Fisheries Centre Research Reports 2011 Volume 19 Number 1 Trade in seahorses and other syngnathids in countries outside Asia (1998-2001) Fisheries Centre, University of British Columbia, Canada Trade in seahorses and other syngnathids in countries outside Asia (1998-2001) 1 Edited by Amanda C.J. Vincent, Brian G. Giles, Christina A. Czembor and Sarah J. Foster Fisheries Centre Research Reports 19(1) 181 pages © published 2011 by The Fisheries Centre, University of British Columbia 2202 Main Mall Vancouver, B.C., Canada, V6T 1Z4 ISSN 1198-6727 1 Cite as: Vincent, A.C.J., Giles, B.G., Czembor, C.A., and Foster, S.J. (eds). 2011. Trade in seahorses and other syngnathids in countries outside Asia (1998-2001). Fisheries Centre Research Reports 19(1). Fisheries Centre, University of British Columbia [ISSN 1198-6727]. Fisheries Centre Research Reports 19(1) 2011 Trade in seahorses and other syngnathids in countries outside Asia (1998-2001) edited by Amanda C.J. Vincent, Brian G. Giles, Christina A. Czembor and Sarah J. Foster CONTENTS DIRECTOR ’S FOREWORD ......................................................................................................................................... 1 EXECUTIVE SUMMARY ............................................................................................................................................. 2 Introduction ..................................................................................................................................................... 2 Methods ........................................................................................................................................................... -

Checklist of the Marine Fishes from Metropolitan France
Checklist of the marine fishes from metropolitan France by Philippe BÉAREZ* (1, 8), Patrice PRUVOST (2), Éric FEUNTEUN (2, 3, 8), Samuel IGLÉSIAS (2, 4, 8), Patrice FRANCOUR (5), Romain CAUSSE (2, 8), Jeanne DE MAZIERES (6), Sandrine TERCERIE (6) & Nicolas BAILLY (7, 8) Abstract. – A list of the marine fish species occurring in the French EEZ was assembled from more than 200 references. No updated list has been published since the 19th century, although incomplete versions were avail- able in several biodiversity information systems. The list contains 729 species distributed in 185 families. It is a preliminary step for the Atlas of Marine Fishes of France that will be further elaborated within the INPN (the National Inventory of the Natural Heritage: https://inpn.mnhn.fr). Résumé. – Liste des poissons marins de France métropolitaine. Une liste des poissons marins se trouvant dans la Zone Économique Exclusive de France a été constituée à partir de plus de 200 références. Cette liste n’avait pas été mise à jour formellement depuis la fin du 19e siècle, © SFI bien que des versions incomplètes existent dans plusieurs systèmes d’information sur la biodiversité. La liste Received: 4 Jul. 2017 Accepted: 21 Nov. 2017 contient 729 espèces réparties dans 185 familles. C’est une étape préliminaire pour l’Atlas des Poissons marins Editor: G. Duhamel de France qui sera élaboré dans le cadre de l’INPN (Inventaire National du Patrimoine Naturel : https://inpn. mnhn.fr). Key words Marine fishes No recent faunistic work cov- (e.g. Quéro et al., 2003; Louisy, 2015), in which the entire Northeast Atlantic ers the fish species present only in Europe is considered (Atlantic only for the former). -

OSTRACIIDAE Boxfishes by K
click for previous page 3948 Bony Fishes OSTRACIIDAE Boxfishes by K. Matsuura iagnostic characters: Small to medium-sized (to 40 cm) fishes; body almost completely encased Din a bony shell or carapace formed of enlarged, thickened scale plates, usually hexagonal in shape and firmly sutured to one another; no isolated bony plates on caudal peduncle. Carapace triangular, rectangular, or pentangular in cross-section, with openings for mouth, eyes, gill slits, pectoral, dorsal, and anal fins, and for the flexible caudal peduncle. Scale-plates often with surface granulations which are prolonged in some species into prominent carapace spines over eye or along ventrolateral or dorsal angles of body. Mouth small, terminal, with fleshy lips; teeth moderate, conical, usually less than 15 in each jaw. Gill opening a moderately short, vertical to oblique slit in front of pectoral-fin base. Spinous dorsal fin absent; most dorsal-, anal-, and pectoral-fin rays branched; caudal fin with 8 branched rays; pelvic fins absent. Lateral line inconspicuous. Colour: variable, with general ground colours of either brown, grey, or yellow, usually with darker or lighter spots, blotches, lines, and reticulations. carapace no bony plates on caudal peduncle 8 branched caudal-fin rays Habitat, biology, and fisheries: Slow-swimming, benthic-dwelling fishes occurring on rocky and coral reefs and over sand, weed, or sponge-covered bottoms to depths of 100 m. Feed on benthic invertebrates. Taken either by trawl, other types of nets, or traps. Several species considered excellent eating in southern Japan, although some species are reported to have toxic flesh and are also able to secrete a substance when distressed that is highly toxic, both to other fishes and themselves in enclosed areas such as holding tanks.