Genetic Diversity of Antarctic Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

Advisory Committee on Fisheries Management
Advisory Committee on Fishery Management ICES CM 2002/ACFM:16 Ref. G REPORT OF THE Working Group on the Biology and Assessment of Deep-Sea Fisheries Resources Horta, the Azores, Portugal 4–10 April 2002 This report is not to be quoted without prior consultation with the General Secretary. The document is a report of an expert group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer Palægade 2–4 DK–1261 Copenhagen K Denmark TABLE OF CONTENTS Section Page 1 INTRODUCTION...................................................................................................................................................... 1 1.1 Terms of Reference......................................................................................................................................... 1 2 OVERVIEW .............................................................................................................................................................. 2 2.1 Background..................................................................................................................................................... 2 2.2 Data availability .............................................................................................................................................. 3 2.3 Ongoing or recently completed research projects/programmes, and activities -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Part 4 Appendices
Part 4 Appendices HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 139 Appendix 1. Proclamation of Heard Island and McDonald Islands Marine Reserve 140 MANAGEMENT PLAN HEARD ISLAND AND MCDONALD ISLANDS MARINE RESERVE 141 142 MANAGEMENT PLAN Appendix 2. Native Fauna of the HIMI Marine Reserve Listed Under the EPBC Act Scientific Name Common Name Birds recorded as breeding Aptenodytes patagonicus king penguin S Catharacta lonnbergi subantarctic skua S Daption capense cape petrel S Diomeda exulans wandering albatross V S M B J A Diomeda melanophrys black–browed albatross S M B A Eudyptes chrysocome southern rockhopper penguin S Eudyptes chrysolophus macaroni penguin S Larus dominicanus kelp gull S Macronectes giganteus southern giant petrel E S M B A Oceanites oceanicus Wilson’s storm petrel S M J Pachyptila crassirostris fulmar prion S Pachyptila desolata Antarctic prion S Pelecanoides georgicus South Georgian diving petrel S Pelecanoides urinatrix common diving petrel S Phalacrocorax atriceps (e) Heard Island cormorant V S Phoebetria palpebrata light mantled sooty albatross S M B A Pygoscelis papua gentoo penguin S Sterna vittata Antarctic tern V S Non–breeding birds Catharacta maccormicki south polar skua S M J Diomedea epomophora southern royal albatross V S M B A Fregetta grallaria white–bellied storm petrel S Fregetta tropica black–bellied storm petrel S Fulmarus glacialoides southern fulmar S Garrodia nereis grey–backed storm petrel S Halobaena caerulea blue petrel V S Macronectes halli northern giant petrel V S M B A Pachyptila belcheri -

Biodiversity of Arctic Marine Fishes: Taxonomy and Zoogeography
Mar Biodiv DOI 10.1007/s12526-010-0070-z ARCTIC OCEAN DIVERSITY SYNTHESIS Biodiversity of arctic marine fishes: taxonomy and zoogeography Catherine W. Mecklenburg & Peter Rask Møller & Dirk Steinke Received: 3 June 2010 /Revised: 23 September 2010 /Accepted: 1 November 2010 # Senckenberg, Gesellschaft für Naturforschung and Springer 2010 Abstract Taxonomic and distributional information on each Six families in Cottoidei with 72 species and five in fish species found in arctic marine waters is reviewed, and a Zoarcoidei with 55 species account for more than half list of families and species with commentary on distributional (52.5%) the species. This study produced CO1 sequences for records is presented. The list incorporates results from 106 of the 242 species. Sequence variability in the barcode examination of museum collections of arctic marine fishes region permits discrimination of all species. The average dating back to the 1830s. It also incorporates results from sequence variation within species was 0.3% (range 0–3.5%), DNA barcoding, used to complement morphological charac- while the average genetic distance between congeners was ters in evaluating problematic taxa and to assist in identifica- 4.7% (range 3.7–13.3%). The CO1 sequences support tion of specimens collected in recent expeditions. Barcoding taxonomic separation of some species, such as Osmerus results are depicted in a neighbor-joining tree of 880 CO1 dentex and O. mordax and Liparis bathyarcticus and L. (cytochrome c oxidase 1 gene) sequences distributed among gibbus; and synonymy of others, like Myoxocephalus 165 species from the arctic region and adjacent waters, and verrucosus in M. scorpius and Gymnelus knipowitschi in discussed in the family reviews. -

Contents of Volume 42 (2012)
CONTENTS OF VOLUME 42 (2012) Issue 1 Tripp-Valdez A., Arreguín-Sánchez F., Zetina-Rejón M.J. The food of Selene peruviana (Actinopterygii: Perciformes: Carangidae) in the southern Gulf of California .................. 1 Zarrad R., Alemany F., Jarboui O., Garcia A., Akrout F. Comparative characterization of the spawning environments of European anchovy, Engraulis encrasicolus , and round sardinella, Sardinella aurita (Actinopterygii: Clupeiformes) in the eastern coast of Tunisia ............................... 9 Ordines F., Valls M., Gouraguine A. Biology, feeding, and habitat preferences of Cadenat’s rockfish, Scorpaena loppei (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Balearic Islands (western Mediterranean) ..................................... 21 Socha M., Sokołowska-Mikołajczyk M., Szczerbik P., Chyb J., Mikołajczyk T., Epler P. The effect of polychlorinated biphenyls mixture (Aroclor 1254) on the embryonic development and hatching of Prussian carp, Carassius gibelio , and common carp, Cyprinus carpio (Actinopterygii: Cypriniformes: Cyprinidae) ............................................................................................................................................................... 31 Khan A., Ghosh K. Characterization and identification of gut-associated phytase-producing bacteria in some fresh water fish cultured in ponds ...................................................................................................................................................................................... -

5Th Indo-Pacific Fish Conference
)tn Judo - Pacifi~ Fish Conference oun a - e II denia ( vernb ~ 3 - t 1997 A ST ACTS Organized by Under the aegis of L'Institut français Société de recherche scientifique Française pour le développement d'Ichtyologie en coopération ' FI Fish Conference Nouméa - New Caledonia November 3 - 8 th, 1997 ABSTRACTS LATE ARRIVAL ZOOLOGICAL CATALOG OF AUSTRALIAN FISHES HOESE D.F., PAXTON J. & G. ALLEN Australian Museum, Sydney, Australia Currently over 4000 species of fishes are known from Australia. An analysis ofdistribution patterns of 3800 species is presented. Over 20% of the species are endemic to Australia, with endemic species occuiring primarily in southern Australia. There is also a small component of the fauna which is found only in the southwestern Pacific (New Caledonia, Lord Howe Island, Norfolk Island and New Zealand). The majority of the other species are widely distributed in the western Pacific Ocean. AGE AND GROWTH OF TROPICAL TUNAS FROM THE WESTERN CENTRAL PACIFIC OCEAN, AS INDICATED BY DAILY GROWm INCREMENTS AND TAGGING DATA. LEROY B. South Pacific Commission, Nouméa, New Caledonia The Oceanic Fisheries Programme of the South Pacific Commission is currently pursuing a research project on age and growth of two tropical tuna species, yellowfm tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). The daily periodicity of microincrements forrned with the sagittal otoliths of these two spceies has been validated by oxytetracycline marking in previous studies. These validation studies have come from fishes within three regions of the Pacific (eastem, central and western tropical Pacific). Otolith microincrements are counted along transverse section with a light microscope. -

Protocols of the EU Bottom Trawl Survey of Flemish Cap
NORTHWEST ATLANTIC FISHERIES ORGANIZATION Scientific Council Studies Number 46 Protocols of the EU bottom trawl survey of Flemish Cap 2014 creative cc commons COMMONS DEED Attribution-NonCommercial 2.5 Canada You are free to copy and distribute the work and to make derivative works under the following conditions: Attribution. You must attribute the work in the manner specified by the author or licensor. Noncommercial. You may not use this work for commercial purposes. Any of these conditions can be waived if you get permission from the copyright holder. Your fair dealing and other rights are in no way affected by the above. http://creativecommons.org/licenses/by/2.5/ca/legalcode.en ISSN-0250-6432 Sci. Council Studies, No. 46, 2014, 1–42 Publication (Upload) date: 21 May 2014 Protocols of the EU bottom trawl survey of Flemish Cap Antonio Vázquez1, José Miguel Casas2 and Ricardo Alpoim3 1Instituto de Investigaciones Marinas, Muelle de Bouzas, Vigo, Spain, Email: [email protected] 2Instituto Español de Oceanografía, Apdo. 1552, 36200 Vigo, Spain, Email: [email protected] 3Instituto Português do Mar e da Atmosfera. Av. Brasília, 1400 Lisboa, Portugal, Email: [email protected] Vázquez, A., J. Miguel Casas, R. Alpoim. 2014. Protocols of the EU bottom trawl survey of Flemish Cap. Scientific Council Studies, 46: 1–42. doi:10.2960/S.v46.m1 Abstract Methods and procedures used in the EU bottom trawl survey of Flemish Cap (NAFO Division 3M) are described in detail. The objectives of publicizing these protocols are to achieve a better understanding of its results, and to contribute to the routines being unaltered. -
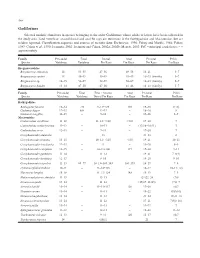
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Genetic Homogeneity in the Deep-Sea Grenadier Macrourus Berglax Across the North Atlantic Ocean
Genetic homogeneity in the deep-sea grenadier Macrourus berglax across the North Atlantic Ocean Coscia, I., Castilho, R., Massa-Gallucci, A., Sacchi, C., Cunha, R. L., Stefanni, S., Helyar, S., Knutsen, H., & Mariani, S. (2018). Genetic homogeneity in the deep-sea grenadier Macrourus berglax across the North Atlantic Ocean. Deep-Sea Research Part I: Oceanographic Research Papers. https://doi.org/10.1016/j.dsr.2017.12.001 Published in: Deep-Sea Research Part I: Oceanographic Research Papers Document Version: Peer reviewed version Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright 2017 Elsevier. This manuscript is distributed under a Creative Commons Attribution-NonCommercial-NoDerivs License (https://creativecommons.org/licenses/by-nc-nd/4.0/), which permits distribution and reproduction for non-commercial purposes, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:02. Oct. -

Zootaxa 3165: 1–24 (2012) ISSN 1175-5326 (Print Edition) Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 3165: 1–24 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) A new species of grenadier, genus Macrourus (Teleostei, Gadiformes, Macrouridae) from the southern hemisphere and a revision of the genus PETER MCMILLAN1, TOMIO IWAMOTO2, ANDREW STEWART3 & PETER J SMITH4 1National Institute of Water and Atmospheric Research Ltd, Private Bag 14901, Wellington, 6241, New Zealand. E-mail: [email protected] 2Department of Ichthyology, California Academy of Sciences, 55 Concourse Drive, San Francisco, CA 94118, USA. E-mail: [email protected] 3Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington, New Zealand 4Museum Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia Abstract A new Macrourus species from the southern hemisphere is described. It was first recognised from the Ross Sea, Antarctica after specimens sampled during the International Polar Year in 2008 showed significant genetic differences (C01) among those initially identified as M. whitsoni (Regan). M. caml sp. nov. has 8 (rarely 7 or 9) pelvic fin rays, a band (2–3 rows) of small uniform-sized teeth in the lower jaw, lacks an outer row of enlarged teeth in the upper jaw, 30–40 scales in a di- agonal row from anal fin origin to lateral line, ventral surface of the head is mostly scaled, except for scaleless areas an- terior to the mouth and on the anterior half of the lower jaw. M caml sp. nov. is large, reaching at least 890 mm TL and appears to be abundant. Numerous specimens caught by commercial bottom longline vessels fishing in the Ross Sea are held at Museum of New Zealand Te Papa Tongarewa, Wellington New Zealand. -

Evolution and Ecology in Widespread Acoustic Signaling Behavior Across Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.14.296335; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Evolution and Ecology in Widespread Acoustic Signaling Behavior Across Fishes 2 Aaron N. Rice1*, Stacy C. Farina2, Andrea J. Makowski3, Ingrid M. Kaatz4, Philip S. Lobel5, 3 William E. Bemis6, Andrew H. Bass3* 4 5 1. Center for Conservation Bioacoustics, Cornell Lab of Ornithology, Cornell University, 159 6 Sapsucker Woods Road, Ithaca, NY, USA 7 2. Department of Biology, Howard University, 415 College St NW, Washington, DC, USA 8 3. Department of Neurobiology and Behavior, Cornell University, 215 Tower Road, Ithaca, NY 9 USA 10 4. Stamford, CT, USA 11 5. Department of Biology, Boston University, 5 Cummington Street, Boston, MA, USA 12 6. Department of Ecology and Evolutionary Biology and Cornell University Museum of 13 Vertebrates, Cornell University, 215 Tower Road, Ithaca, NY, USA 14 15 ORCID Numbers: 16 ANR: 0000-0002-8598-9705 17 SCF: 0000-0003-2479-1268 18 WEB: 0000-0002-5669-2793 19 AHB: 0000-0002-0182-6715 20 21 *Authors for Correspondence 22 ANR: [email protected]; AHB: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.14.296335; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.