Chemical Compatibility Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Dissertation Has Been 62—2136 M Icrofilm Ed Exactly As Received GIELISSE, Peter Jacob M., 1934- INVESTIGATION of PHASE EQ
This dissertation has been 62—2136 microfilmed exactly as received GIELISSE, Peter Jacob M., 1934- INVESTIGATION OF PHASE EQUILIBRIA IN THE SYSTEM ALUMINA-BORON OXIDE-SILICA. The Ohio State University, Ph.D., 1961 M ineralogy University Microfilms, Inc., Ann Arbor, Michigan INVESTIGATION OP PHASE EQUILIBRIA IN THE SYSTEM ALUMINA-BORON OXIDE-SILICA DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Peter Jacob M. Gielisse, M. S. The Ohio State University 1961 Approved by Adviser Department of Mineralogy ACKNOWLEDGMENTS The writer wishes to extend his sincere thanks to the many people without whose help the preparation of this dissertation would have been impossible. He is indebted in particular to his adviser, Dr. Wilfrid R. Foster, for his invaluable aid, advice and many kindnesses; to the other members of the faculty of the Department of Mineral ogy, Drs. Ernest G. Ehlers, Henry E. Wenden, and Rodney T Tettenhorst; and to his friend and colleague, Thomas J. Rockett. Acknowledgment is also made for financial support re ceived under contract No. AF 33(616)-3189, sponsored by Aeronautical Research Laboratories, Air Force Research Division, Wright Patterson Air Force Base, Ohio; as well as for aid received through a Mershon National Graduate Fellowship awarded to the writer by the Mershon Committee on Education in National Security for 1960-‘61'. It goes without saying that he is also most grate ful to his wife, Anna, for her excellent help and encour agement over the years. TABLE OF CONTENTS Page INTRODUCTION ...................................... -

Implementation of Mathematical Equation for Calculating Alumina Extraction from Bauxite Tailing Digestion
International Conference on Applied Mathematics, Simulation and Modelling (AMSM 2016) Implementation of Mathematical Equation for Calculating Alumina Extraction from Bauxite Tailing Digestion Saini Hu Mineral and Coal Technology Research and Development CenterJalan Jenderal Sudirman No. 623 Bandung-Indonesia Abstract—Research on bauxite digesting using pressurized Washing is one of methods that are usually used to upgrade reactor at a capacity of 86,66 kg of feed/batch had been alumina content of bauxite by separating primary mineral conducted. Bauxite with -150 mesh of particle size is reacted impurities like clay, silica, iron, and titanium that are with 42,15 kg of caustic soda with concentration of 433,49 g/l at attached on the surface of crude bauxite [3]. Trommol screen the temperature of 140oC for 1.0 to 2.5 hours using steam as is common equipment to wash and screen coarse bauxite heating media. Lime added are varied from 3 to 9 kg. After from very fine particles size by supporting pressurized spray processing for a certain period of time, slurry product is water. By using water spraying and screening, smaller transferred into a Mixer. To evaluate percent yield of Al2O3 particles attached on the surface of crude bauxite is easy to extraction from this process, measuring the height of slurry separate. Therefore, there are two products of washing, level in the Mixer, densities of the slurry, filtrate, and solid namely washed bauxite having +2mm of particle size with residue are conducted. Head sample of bauxite, filtrate and high content of alumina and tailing having –2mm of particle residue are analysed by using wet method to get Al2O3 content size with lower content of alumina. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

(12) Patent Application Publication (10) Pub. No.: US 2011/005250.6 A1 Abel Et Al
US 2011 0052506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/005250.6 A1 Abel et al. (43) Pub. Date: Mar. 3, 2011 (54) STABLE AEROSOL TOPICAL FOAMS Publication Classification COMPRISINGA HYPOCHLORITE SALT (51) Int. Cl. (75) Inventors: Douglas Abel, Sudbury, MA (US); st 94;O CR Ronald M. Gurge, Franklin, MA ( .01) (US); Mark W. Trumbore, A6IP3L/00 (2006.01) Westford, MA (US) (52) U.S. Cl. .......................................................... 424/45 (73) Assignee: Collegium Pharmaceutical, Inc., (57) ABSTRACT Cumberland, RI (US) Described herein are compositions useful in the treatment of atopic dermatitis and other skin conditions, which composi (21) Appl. No.: 12/872,566 tions exhibit enhanced stability. The compositions contain a 1-1. hypochlorite salt, useful for its antimicrobial properties, and (22) Filed: Aug. 31, 2010 are non-irritating when applied to the skin. The compositions O O also provide enhanced moisturizing properties. The compo Related U.S. Application Data sitions can be formulated into a topical aerosol foam with (60) Provisional application No. 61/238,439, filed on Aug. inert, non-flammable propellants, such as hydrofluoroal 31, 2009. kanes, and may be used in cosmetics or pharmaceuticals. US 2011/005250.6 A1 Mar. 3, 2011 STABLE AEROSOL TOPCAL FOAMS itch associated with atopic dermatitis; however, they can COMPRISINGA HYPOCHLORITE SALT cause sleepiness and may not help in all cases of atopic dermatitis. RELATED APPLICATION 0007 For mild cases of atopic dermatitis, an over-the counterformulation of coal taris often used. Coaltar has long 0001. This application claims benefit under 35 U.S.C. 119 been a treatment for a variety of skin conditions. -

Rubber and Composite Hose Chemical Resistant Chart
Rubber and Composite Hose Chemical Resistant Chart Cedar Rapids, IA Corporate Headquarters Phone: 319.365.0471 Toll Free: 800.553.5455 Fax: 319.365.2522 Website: www.apache-inc.com 99004032 rev032713 Chemical Resistance Information This Apache document provides essential information that will facilitate the safe use of rubber and composite type chemical hoses. Chemical hose users are cautioned that this Chemical Resistant has be developed from generallty accepted industry standards. The ratings listed beneath each Elastomer are the base ratings for the chemical listed. This rating is based on the application temperature not exceeding 70ºF (21.1ºC) unless otherwise specified. The degree an Elastomer will resist the effects of a of a specific chemical depends on several variables.It is recommended that a hose with the highest resistant tube to the chemical transferred be used in the application for safety. 1. Concentration of the chemical is very significant (some chemicals may react with an Elastomer differently based on the level of concentration). 2. Temperature - as the temperature increases the deteriorative effect of a chemical may greatly increase on an Elastomer. 3. Time - the longer the duration the chemical is in contact with the Elastomer, the greater the deteriorative effect. 4. Stability of the Chemical - Chemical solutions (combining of different chemicals) may increase the deteriorative effect. 5. Elastomer Grade - There are different grades of specific Elastomer used in hose. The grade of Elastomer used may effect the resistance level of the hose to a specific chemical. It is recommended that only hose listed for chemical service be use. 6. Safety a. -

Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According to Federal Register / Vol
Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations And According To The Hazardous Products Regulation (February 11, 2015). Revision Date: 04/03/2019 Date of Issue: 01/22/2014 Supersedes Date: 03/08/2017 Version: 2.1 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Mixture Product Name: Ammonium Thiosulfate, Solution ((NH4)2S2O3) Synonyms: Ammonium Hyposulfite, Solution 1.2. Intended Use of the Product No use is specified. 1.3. Name, Address, and Telephone of the Responsible Party Company Manufacturer Poole Chemical Co., Inc. Poole Chemical Co., Inc. P.O. Box 10 P.O. Box 10 100 N. 1st Street 100 N. 1st Street Texline, TX 79087 - United States Texline, TX 79087 - United States T 806-362-4261 T 806-362-4261 1.4. Emergency Telephone Number Emergency Number : 1-800-424-9300 (CHEMTREC) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture GHS-US/CA Classification Not classified 2.2. Label Elements GHS-US/CA Labeling No labeling applicable 2.3. Other Hazards Exposure may aggravate pre-existing eye, skin, or respiratory conditions. 2.4. Unknown Acute Toxicity (GHS-US/CA) No data available SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.1. Substance Not applicable 3.2. Mixture Name Synonyms Product Identifier % * GHS Ingredient Classification Ammonium thiosulfate Ammonium thiosulphate / Thiosulfuric acid, (CAS-No.) 7783-18-8 60 Not classified diammonium salt / Thiosulfuric acid (H2S2O3), diammonium salt / Thiosulfuric acid (H2S2O3), ammonium salt (1:2) / Diammonium thiosulfate Water AQUA / Aqua (CAS-No.) 7732-18-5 40 Not classified *Percentages are listed in weight by weight percentage (w/w%) for liquid and solid ingredients. -

Investigation of Lime Usage Impacts on Bauxite Processability at ETI Aluminyum Plant
International Journal of Industrial Chemistry (2019) 10:57–66 https://doi.org/10.1007/s40090-019-0171-x RESEARCH Investigation of lime usage impacts on bauxite processability at ETI Aluminyum Plant Hüseyin Arıkan1 · Gökhan K. Demir2 · Sema Vural3 Received: 29 March 2018 / Accepted: 3 February 2019 / Published online: 11 February 2019 © The Author(s) 2019 Abstract ETI Aluminyum A.Ş., the primary aluminium manufacturer of Turkey, is also one of the major integrated plants of the world capable of performing production from mining until end product. The alumina refnery was designed on a certain boehmitic bauxite quality basis. However, bauxite properties have changed over the years, resulting in the urgent need for process opti- mization to not only keep the refnery cost efcient, but also prevent serious bottlenecks in the near future. Lime can be used to overcome problems when using bauxites with specifc and variable chemical and mineralogical characteristics. Although lime is extensively used when processing diasporic bauxites, the studies on boehmitic bauxites and the overall efects on the process are limited. In this paper, lime impact was investigated at all stages of the Bayer process including the efect on alumina quality, energy and raw material consumptions. The results showed signifcant improvements in the product quality as well as recordable savings on consumptions. Keywords Bayer process · Lime usage · Caustic consumption · Digestion · Alumina quality · Boehmitic bauxite Introduction crystalline structure to boehmite. Diferences in ore compo- sition and presence of iron, silicon and titanium impurities The Bayer process is used for producing alumina (Al2O3) infuence their subsequent processing [3, 4]. Thus, it is nec- from bauxite ore. -
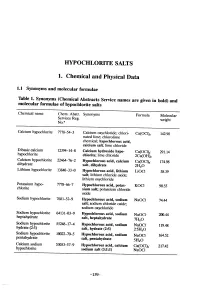
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Inventory of Biocides Used in Denmark
Environmental Project No. 585 2001 Miljøprojekt Inventory of Biocides used in Denmark Carsten Lassen, Susanne Skårup, Sonja Hagen Mikkelsen og Jesper Kjølholt COWI Pia Juul Nielsen Danish Toxicology Centre Lise Samsøe-Petersen DHI - Water & Environment The Danish Environmental Protection Agency will, when opportunity offers, publish reports and contributions relating to environmental research and development projects financed via the Danish EPA. Please note that publication does not signify that the contents of the reports necessarily reflect the views of the Danish EPA. The reports are, however, published because the Danish EPA finds that the studies represent a valuable contribution to the debate on environmental policy in Denmark. Table of Contents SUMMARY 5 DANSK SAMMENFATNING 9 1 INTRODUCTION 13 2 METHODOLOGY 15 3 MAIN GROUP 1: DISINFECTANTS AND GENERAL BIOCIDAL PRODUCTS 18 3.1 PRODUCT-TYPE 1: HUMAN HYGIENE BIOCIDAL PRODUCTS 18 3.1.1 Skin disinfectants 19 3.2 PRODUCT-TYPE 2: PRIVATE AREA AND PUBLIC HEALTH AREA DISINFECTANTS AND OTHER BIOCIDAL PRODUCTS 23 3.2.1 Disinfectants for private areas 23 3.2.2 Disinfectants for professional cleaning and industrial use 24 3.2.3 Disinfectants for medical equipment 27 3.2.4 Disinfectants for laundries 29 3.2.5 Disinfectants for air-conditioning system 30 3.2.6 Disinfectants for chemical toilets 30 3.2.7 Disinfectants for swimming pools 31 3.2.8 Disinfectants for wastewater and hospital waste 32 3.3 PRODUCT-TYPE 3: VETERINARY HYGIENE BIOCIDAL PRODUCTS 33 3.3.1 Disinfectants applied directly to -

Ammonium Thiosulfate 12-0-0, 26% Sulfur
Secondary Nutrients Ammonium Thiosulfate 12-0-0, 26% Sulfur Guaranteed Analysis Directions for Use: Greens, Tees and Fine Turf: Apply 3.0 - 12.0 oz. of Ammonium Thiosulfate with 1.5 -2 gallons of Total Nitrogen (N) ................................... 12.00% water per 1,000 sq. ft. (1.0 - 4.1 gallons of Ammonium Thiosulfate with 66 - 88 gallons of water per 12% Ammoniacal Nitrogen Sulfur (S) ................................................. 26.00% Acre) every 14 days throughout the growing season. Irrigate after application. This application shall provide 0.03 - 0.12 lb. of actual Nitrogen per 1,000 sq. ft. Derived from Ammonium Thiosulfate Fairways, Roughs, Sports Turf and Lawns: Apply 3.1 - 4.1 gallons of Ammonium Thiosulfate Ammonium Thiosulfate is an excellent source with 44 - 88 gallons of water per Acre (9.0 - 12.0 oz. of Ammonium Thiosulfate with 1 - 2 gallons of of ammoniacal nitrogen that is quickly absorbed water per 1,000 sq. ft.) every 14 days throughout the growing season. This application shall provide by the plant. This results in greener turf, even at 0.09 - 0.12 lb. of actual nitrogen per 1,000 sq. ft. low soil temperatures. Use when a liquid type of ammoniacal nitrogen source and sulfur are Fertigation: Apply 1.0 - 5.0 gallons per Acre (3 - 15 oz. per 1,000 sq. ft.) of Ammonium Thiosulfate required. with the irrigation water every 7 to 14 throughout the growing season. Ammonium Thiosulfate is a neutral to slightly Application Precautions: basic (7 - 8 pH), clear liquid solution, containing 12% nitrogen and 26% sulfur. Ammonium Do not apply Ammonium Thiosulfate directly on or below germinating seeds such as in a “pop Thiosulfate is compatible with most liquid up” fertilizer. -

Orca Corrosion Chart
Unsaturated Polyester Vinylster (Epoxy Acrylate Resins) CHEMICAL Conc Resins NO ISO BIS Novolac Bromine ENVIRONMENT % 511/512 301 585 570 545/555 A 1 Acetaldehyde 20 NR 40 40 40 2 Acetic Acid 10 80 100 100 100 3 Acetic Acid 15 60 100 100 100 4 Acetic Acid 25 60 100 100 100 5 Acetic Acid 50 - 80 80 80 6 Acetic Acid 75 NR 65 65 65 7 Acetic Acid, Glacial 100 NR NR 40 NR 8 Acetic Anhydride 100 NR NR 40 NR 9 Acetone 10 NR NR 80 80 10 Acetone 100 NR NR NR NR 11 Acetonitrile 20 - 40 40 40 12 Acetyl Acetone 20 - 40 50 40 13 Acrolein (Acrylaldehyde) 20 - 40 40 40 14 Acrylamide 50 NR 40 40 40 15 Acrylic Acid 25 NR 40 40 40 16 Acrylic Latex All - 80 80 80 17 Acrylonitrile Latex Dispersion 2 NR 25 25 25 Activated Carbon Beds, Water 18 - 80 100 80 Treatment Adipic Acid(1.5g solution in 19 23 - 80 80 80 water at 25℃, sol in hot water) 20 ALAMINE amines - 65 80 65 21 Alkyl(C8-10) Dimethyl Amine 100 - 80 100 80 22 Alkyl(C8-10) Chloride All - 80 100 95 23 Alkyl Benzene Sulfonic Acid 90 NR 50 50 50 Alkyl Tolyl Trimethyl 24 - - 40 50 40 Ammonium Chloride 25 Allyl Alcohol 100 NR NR 25 NR 26 Allyl Chloride All NR 25 25 25 27 Alpha Methylstyrene 100 NR 25 50 25 28 Alpha Oleum Sulfates 100 NR 50 50 50 29 Alum Sat'd 80 100 120 100 30 Aluminum Chloride Sat'd 80 100 120 100 31 Aluminum Chlorohydrate All - 100 100 100 32 Aluminum Chlorohydroxide 50 - 100 100 100 33 Aluminum Fluoride All - 25 25 25 34 Aluminum Hydroxide 100 80 80 95 80 35 Aluminum Nitrate All 80 100 100 100 36 Aluminum Potassium Sulfate Sat'd 80 100 120 100 37 Aluminum Sulfate Sat'd 80 100 120 100