ATS: the Multi-Use Sulfur Fertilizer Researcher Cites Data That Demonstrate the Muli-Benefits of This Valuable Sulfur Source
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According to Federal Register / Vol
Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations And According To The Hazardous Products Regulation (February 11, 2015). Revision Date: 04/03/2019 Date of Issue: 01/22/2014 Supersedes Date: 03/08/2017 Version: 2.1 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Mixture Product Name: Ammonium Thiosulfate, Solution ((NH4)2S2O3) Synonyms: Ammonium Hyposulfite, Solution 1.2. Intended Use of the Product No use is specified. 1.3. Name, Address, and Telephone of the Responsible Party Company Manufacturer Poole Chemical Co., Inc. Poole Chemical Co., Inc. P.O. Box 10 P.O. Box 10 100 N. 1st Street 100 N. 1st Street Texline, TX 79087 - United States Texline, TX 79087 - United States T 806-362-4261 T 806-362-4261 1.4. Emergency Telephone Number Emergency Number : 1-800-424-9300 (CHEMTREC) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture GHS-US/CA Classification Not classified 2.2. Label Elements GHS-US/CA Labeling No labeling applicable 2.3. Other Hazards Exposure may aggravate pre-existing eye, skin, or respiratory conditions. 2.4. Unknown Acute Toxicity (GHS-US/CA) No data available SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.1. Substance Not applicable 3.2. Mixture Name Synonyms Product Identifier % * GHS Ingredient Classification Ammonium thiosulfate Ammonium thiosulphate / Thiosulfuric acid, (CAS-No.) 7783-18-8 60 Not classified diammonium salt / Thiosulfuric acid (H2S2O3), diammonium salt / Thiosulfuric acid (H2S2O3), ammonium salt (1:2) / Diammonium thiosulfate Water AQUA / Aqua (CAS-No.) 7732-18-5 40 Not classified *Percentages are listed in weight by weight percentage (w/w%) for liquid and solid ingredients. -

Ammonium Thiosulfate 12-0-0, 26% Sulfur
Secondary Nutrients Ammonium Thiosulfate 12-0-0, 26% Sulfur Guaranteed Analysis Directions for Use: Greens, Tees and Fine Turf: Apply 3.0 - 12.0 oz. of Ammonium Thiosulfate with 1.5 -2 gallons of Total Nitrogen (N) ................................... 12.00% water per 1,000 sq. ft. (1.0 - 4.1 gallons of Ammonium Thiosulfate with 66 - 88 gallons of water per 12% Ammoniacal Nitrogen Sulfur (S) ................................................. 26.00% Acre) every 14 days throughout the growing season. Irrigate after application. This application shall provide 0.03 - 0.12 lb. of actual Nitrogen per 1,000 sq. ft. Derived from Ammonium Thiosulfate Fairways, Roughs, Sports Turf and Lawns: Apply 3.1 - 4.1 gallons of Ammonium Thiosulfate Ammonium Thiosulfate is an excellent source with 44 - 88 gallons of water per Acre (9.0 - 12.0 oz. of Ammonium Thiosulfate with 1 - 2 gallons of of ammoniacal nitrogen that is quickly absorbed water per 1,000 sq. ft.) every 14 days throughout the growing season. This application shall provide by the plant. This results in greener turf, even at 0.09 - 0.12 lb. of actual nitrogen per 1,000 sq. ft. low soil temperatures. Use when a liquid type of ammoniacal nitrogen source and sulfur are Fertigation: Apply 1.0 - 5.0 gallons per Acre (3 - 15 oz. per 1,000 sq. ft.) of Ammonium Thiosulfate required. with the irrigation water every 7 to 14 throughout the growing season. Ammonium Thiosulfate is a neutral to slightly Application Precautions: basic (7 - 8 pH), clear liquid solution, containing 12% nitrogen and 26% sulfur. Ammonium Do not apply Ammonium Thiosulfate directly on or below germinating seeds such as in a “pop Thiosulfate is compatible with most liquid up” fertilizer. -

Orca Corrosion Chart
Unsaturated Polyester Vinylster (Epoxy Acrylate Resins) CHEMICAL Conc Resins NO ISO BIS Novolac Bromine ENVIRONMENT % 511/512 301 585 570 545/555 A 1 Acetaldehyde 20 NR 40 40 40 2 Acetic Acid 10 80 100 100 100 3 Acetic Acid 15 60 100 100 100 4 Acetic Acid 25 60 100 100 100 5 Acetic Acid 50 - 80 80 80 6 Acetic Acid 75 NR 65 65 65 7 Acetic Acid, Glacial 100 NR NR 40 NR 8 Acetic Anhydride 100 NR NR 40 NR 9 Acetone 10 NR NR 80 80 10 Acetone 100 NR NR NR NR 11 Acetonitrile 20 - 40 40 40 12 Acetyl Acetone 20 - 40 50 40 13 Acrolein (Acrylaldehyde) 20 - 40 40 40 14 Acrylamide 50 NR 40 40 40 15 Acrylic Acid 25 NR 40 40 40 16 Acrylic Latex All - 80 80 80 17 Acrylonitrile Latex Dispersion 2 NR 25 25 25 Activated Carbon Beds, Water 18 - 80 100 80 Treatment Adipic Acid(1.5g solution in 19 23 - 80 80 80 water at 25℃, sol in hot water) 20 ALAMINE amines - 65 80 65 21 Alkyl(C8-10) Dimethyl Amine 100 - 80 100 80 22 Alkyl(C8-10) Chloride All - 80 100 95 23 Alkyl Benzene Sulfonic Acid 90 NR 50 50 50 Alkyl Tolyl Trimethyl 24 - - 40 50 40 Ammonium Chloride 25 Allyl Alcohol 100 NR NR 25 NR 26 Allyl Chloride All NR 25 25 25 27 Alpha Methylstyrene 100 NR 25 50 25 28 Alpha Oleum Sulfates 100 NR 50 50 50 29 Alum Sat'd 80 100 120 100 30 Aluminum Chloride Sat'd 80 100 120 100 31 Aluminum Chlorohydrate All - 100 100 100 32 Aluminum Chlorohydroxide 50 - 100 100 100 33 Aluminum Fluoride All - 25 25 25 34 Aluminum Hydroxide 100 80 80 95 80 35 Aluminum Nitrate All 80 100 100 100 36 Aluminum Potassium Sulfate Sat'd 80 100 120 100 37 Aluminum Sulfate Sat'd 80 100 120 100 -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1) CHEMICAL PRODUCT AND SUPPLIER'S IDENTIFICATION Product ID: Ammonium Thiosulfate Product Name: Ammonium Thiosulfate Revision Date: Jul 13, 2015 Date Printed: Sep 29, 2015 Version: 2.0 Supersedes Date: Jun 05, 2015 Manufacturer's Name: Martin Operating Partnership, L.P. Address: P.O. Box 191, Kilgore, TX, US, 75663 Emergency Phone: CHEMTREC (800) 424-9300 Information Phone: 800-231-4595 Fax: Product/Recommended Uses: Industrial uses SECTION 2) HAZARDS IDENTIFICATION Classification: Acute toxicity, Oral - Category 4 Pictograms: Signal Word: Warning Hazardous Statements - Health: Harmful if swallowed Precautionary Statements - General: If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. Precautionary Statements - Prevention: Wash with soap and water thoroughly after handling. Do not eat, drink or smoke when using this product. Precautionary Statements - Response: IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. Precautionary Statements - Storage: No precautionary statement available. Precautionary Statements - Disposal: Dispose of contents/container to disposal recycling center. Under RCRA it is the responsibility of the user of the product to determine at the time of disposal whether the product meets RCRA criteria for hazardous waste. Waste management should be in full compliance with federal, state and local laws. Ammonium Thiosulfate www.martinresources.com Page 1 of 7 SECTION 3) COMPOSITION / INFORMATION ON INGREDIENTS CAS Chemical Name % By Weight 0007783-18-8 AMMONIUM THIOSULFATE 48% - 66% 0007732-18-5 WATER 34% - 46% 0007783-20-2 AMMONIUM SULFATE 0.9% - 2% 0010196-04-0 AMMONIUM SULFITE 0.1% - 2% SECTION 4) FIRST-AID MEASURES Inhalation: Remove source of exposure or move person to fresh air and keep comfortable for breathing. -

Effect of Surface Application of Ammonium Thiosulfate on Field
Science of the Total Environment 580 (2017) 316–323 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Effect of surface application of ammonium thiosulfate on field-scale emissions of 1,3-dichloropropene S.R. Yates a,⁎, D.J. Ashworth a,b,Q.Zhanga a USDA-ARS, U.S. Salinity Laboratory, 450 W. Big Springs Rd., Riverside, CA 92507, United States b University of California, Department of Environmental Sciences, Riverside, CA 92521, United States HIGHLIGHTS GRAPHICAL ABSTRACT • Five methods and two independent da- ta sets were used to calculate emissions • Total 1,3-dichloropropene mass loss was 18.4 ± 6.7% and ranged 12–26% • Spraying ammonium thiosulfate fertil- izer on soil reduced 1,3-D emissions • Results compared to four related large- scale field experiments article info abstract Article history: Soil fumigation is important for food production but has the potential to discharge toxic chemicals into the environ- Received 29 September 2016 ment, which may adversely affect human and ecosystem health. A field experiment was conducted to evaluate the Received in revised form 17 November 2016 effect of applying ammonium thiosulfate fertilizer to the soil surface prior to fumigating with 1,3-dichloropropene Accepted 17 November 2016 (1,3-D). The ammonium thiosulfate solution was applied as a spray with minimal water to minimize the effect on Available online 21 December 2016 emissions from saturating (e.g. sealing) the soil pores with water. Two independent data sets were collected for de- Editor: Jay Gan termining the emission rate. One data set was used with three micrometeorological approaches: aerodynamic, inte- grated horizontal flux and theoretical profile shape; the other dataset with two indirect, back calculation methods Keywords: that used the CALPUFF and ISCST3 dispersion models. -

Ammonium Thiosulfate MSDS
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard 0 1 1 0 Fire Hazard 0 Reactivity 0 See Section 15. Section 1. Chemical Product and Company Identification Page Number: 1 Common Name/ Ammonium Thiosulfate Catalog A1273 Trade Name Number(s). CAS# 7783-18-8 Manufacturer SPECTRUM LABORATORY PRODUCTS INC. RTECS XN6465000 14422 S. SAN PEDRO STREET TSCA TSCA 8(b) inventory: GARDENA, CA 90248 Ammonium Thiosulfate Commercial Name(s) Thio-sul CI# Not available. Synonym Diammonium Thiosulfate; Ammonium Hyposulfite IN CASE OF EMERGENCY Chemical Name Thiosulfuric acid, diammonium salt CHEMTREC (24hr) 800-424-9300 Chemical Family Not available. CALL (310) 516-8000 Chemical Formula H8-N2.O3-S2 Supplier SPECTRUM LABORATORY PRODUCTS INC. 14422 S. SAN PEDRO STREET GARDENA, CA 90248 Section 2.Composition and Information on Ingredients Exposure Limits Name CAS # TWA (mg/m3) STEL (mg/m3) CEIL (mg/m3) % by Weight 1) Ammonium Thiosulfate{2} 7783-18-8 100 Toxicological Data Not applicable. on Ingredients Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Potential Chronic Health CARCINOGENIC EFFECTS: Not available. Effects MUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure is not known to aggravate medical condition. Continued on Next Page Ammonium Thiosulfate Page Number: 2 Section 4. First Aid Measures Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention if irritation occurs. -

Exhibit 2D-3
Exhibit 2D–3. Hazardous Substances 1. Acetaldehyde 73. Captan 144. Ferrous sulfate 2. Acetic acid 74. Carbaryl 145. Formaldehyde 3. Acetic anhydride 75. Carbofuran 146. Formic acid 4. Acetone cyanohydrin 76. Carbon disulfide 147. Fumaric acid 5. Acetyl bromide 77. Carbon tetrachloride 148. Furfural 6. Acetyl chloride 78. Chlordane 149. Guthion 7. Acrolein 79. Chlorine 150. Heptachlor 8. Acrylonitrile 80. Chlorobenzene 151. Hexachlorocyclopentadiene 9. Adipic acid 81. Chloroform 152. Hydrochloric acid 10. Aldrin 82. Chloropyrifos 153. Hydrofluoric acid 11. Allyl alcohol 83. Chlorosulfonic acid 154. Hydrogen cyanide 12. Allyl chloride 84. Chromic acetate 155. Hydrogen sulfide 13. Aluminum sulfate 85. Chromic acid 156. Isoprene 14. Ammonia 86. Chromic sulfate 157. Isopropanolamine dodecylbenzenesulfonate 15. Ammonium acetate 87. Chromous chloride 158. Kelthane 16. Ammonium benzoate 88. Cobaltous bromide 159. Kepone 17. Ammonium bicarbonate 89. Cobaltous formate 160. Lead acetate 18. Ammonium bichromate 90. Cobaltous sulfamate 161. Lead arsenate 19. Ammonium bifluoride 91. Coumaphos 162. Lead chloride 20. Ammonium bisulfite 92. Cresol 163. Lead fluoborate 21. Ammonium carbamate 93. Crotonaldehyde 164. Lead fluorite 22. Ammonium carbonate 94. Cupric acetate 165. Lead iodide 23. Ammonium chloride 95. Cupric acetoarsenite 166. Lead nitrate 24. Ammonium chromate 96. Cupric chloride 167. Lead stearate 25. Ammonium citrate 97. Cupric nitrate 168. Lead sulfate 26. Ammonium fluoroborate 98. Cupric oxalate 169. Lead sulfide 27. Ammonium fluoride 99. Cupric sulfate 170. Lead thiocyanate 28. Ammonium hydroxide 100. Cupric sulfate ammoniated 171. Lindane 29. Ammonium oxalate 101. Cupric tartrate 172. Lithium chromate 30. Ammonium silicofluoride 102. Cyanogen chloride 173. Malathion 31. Ammonium sulfamate 103. Cyclohexane 174. Maleic acid 32. Ammonium sulfide 104. -

CHEMICAL RESISTANCE CHART Chemical Resistance Glove Chart
CHEMICAL RESISTANCE CHART Chemical Resistance Glove Chart P = Poor | F = Fair | G = Good | E = Excellent | X = Not Tested Chemical Latex Nitrile 1,1,1,2-Tetrachloroethane P F P 1,1,1-Trichloroethane P P P 1,1,2-Trichloroethane P P P 1,2,4,5-Tetrachlorobenzene X E P 1,2,4-Trichlorobenzene P F P 1,2-Dichlorobenzene (o-Dichlorobenzene) P P P 1,2-Dichloroethylene P P P 1,2-Propylenoxide P P P 1,3-Dioxane F P P 1,4-Dioxane P P P 1-Nitropropane G P P 2-(Dietylamino)ethanol P E P 2-Chloroethanol X P P 2-Hydroxyethyl acrylate P P P 2-Hydroxyethyl methacrylate (HEMA) X X X 2-Nitropropane P P P 2-Nitrotoluene P P P 3-Bromopropionic acid G F X 4,4-Methylenedianiline (MDA) X X X 5-Fluorouracil X X X Acetaldehyde G P P Acetamide G P P Acetate solvent G F P Acetic acid (glacial acetic acid) 99+ % G F F Acetic acid 30% E G F Acetic acid anhydride G F P Acetone F P P Acetonitrile F F P Acetyl chloride P P P Acetylene G E F Acryl Acid P F P Acrylamide, 30-70% P E F Acrylic Acid G G P Acrylonitrile P P P Adipic acid E E X Allyl alcohol P P P Allylamine P P P Allylchloride (3-Chloroporpene) P P P Aluminium Acetate E G X Aluminium Chloride E E G Aluminium Fluoride G E F Aluminium Hydroxide P E F Aluminium Nitrate E E X Aluminium Potassium Sulfate E E F Aluminium Sulfate E E G Amine G P Ammonia anhdyrous P G G Ammonia gas (cold) E E X Ammonia gas (hot) P P X Ammonia Nitrate X F X 74 Chemical Resistance Glove Chart P = Poor | F = Fair | G = Good | E = Excellent | X = Not Tested Chemical Latex Nitrile Ammonia solution, 30% P E X Ammonium Acetate E E X Ammonium -

Click Here (PDF)
Chemical Products Caustic Soda, Liquid (Gluconated) Fumaric Acid Monoaluminum Phosphate Cetyl Alcohol Monoammonium Phosphate Acetic Acid Chromic Acid Monocalcium Phosphate Acetic Anhydride CIP Cleaner Monoethanolamine (MEA) Acetone Citric Acid (Dry or Liquid) Gluconate Liquid Monopotassium Phosphate Activated Carbon Cocamide DEA Gluconic Acid Monosodium Glutamate (MSG) Alcohol Ethoxylates (91-6, 91-8, etc) Cocamide MEA Glycerine (Crude, Tech, or USP) Monosodium Phosphate Alkanolamides Cocamidopropyl Betaine Glycol Ether, “E” Series Muriate of Potash (KCL) Alkyl Ether Sulfates Copper Cyanide Glycol Ether, “P” Series Muriatic Acid (HCL) Alkyl Sulfates Copper Nitrate Glycol Stearate Aloe Vera Copper Sulfate Glycolic Acid Alpha Methyl Styrene (AMS) Cosmetic Oils Aluminum Chloride Cyclohexane Nickel Chloride Aluminum Stearate Cyclohexanone Nickel Sulfate Aluminum Sulfate (Dry or Liquid) Cyclohexylamine Heptane Nitric Acid Amido-Amines Hexane N-Methyl Pyrrolidone Amine Oxides Hexylene Glycol NPE Surfactant (NP-4, NP-6, NP-9) Ammonia, Aqua Hydrazine N-Propyl Acetate Ammonium Bifluoride Dequest® Hydrochloric Acid N-Propyl Alcohol Ammonium Chloride Diacetone Alcohol (Inhibited Available) NTA Ammonium Lauryl Sulfate Diammonium Phosphate Hydrofluoric Acid Ammonium Persulfate Diatomaceous Earth Hydrofluosilicic Acid Ammonium Sulfate Dibasic Esters Hydrogen Peroxide Ammonium Thioglycolate Dicalcium Phosphate Hydroxyacetic Acid Oxalic Acid Ammonium Thiosulfate Dicalite® Hydroxylamine Sulfate Amphoterics Diethanolamine (DEA) Hypophosphorous Acid Amyl -
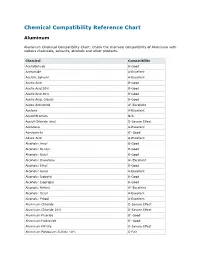
Chemical Compatibility Reference Chart
Chemical Compatibility Reference Chart Aluminum Aluminum Chemical Compatibility Chart: Check the chemical compatibility of Aluminum with various chemicals, solvents, alcohols and other products. Chemical Compatibility Acetaldehyde B-Good Acetamide A-Excellent Acetate Solvent A-Excellent Acetic Acid B-Good Acetic Acid 20% B-Good Acetic Acid 80% B-Good Acetic Acid, Glacial B-Good Acetic Anhydride A1-Excellent Acetone A-Excellent Acetyl Bromide N/A Acetyl Chloride (dry) D-Severe Effect Acetylene A-Excellent Acrylonitrile B1-Good Adipic Acid A-Excellent Alcohols: Amyl B-Good Alcohols: Benzyl B-Good Alcohols: Butyl B-Good Alcohols: Diacetone A1-Excellent Alcohols: Ethyl B-Good Alcohols: Hexyl A-Excellent Alcohols: Isobutyl B-Good Alcohols: Isopropyl B-Good Alcohols: Methyl A1-Excellent Alcohols: Octyl A-Excellent Alcohols: Propyl A-Excellent Aluminum Chloride D-Severe Effect Aluminum Chloride 20% D-Severe Effect Aluminum Fluoride B1-Good Aluminum Hydroxide B1-Good Aluminum Nitrate D-Severe Effect Aluminum Potassium Sulfate 10% C-Fair Aluminum Potassium Sulfate 100% C-Fair Aluminum Sulfate B1-Good Alums A-Excellent Amines B-Good Ammonia 10% A2-Excellent Ammonia Nitrate C-Fair Ammonia, anhydrous A1-Excellent Ammonia, liquid A-Excellent Ammonium Acetate A-Excellent Ammonium Bifluoride B-Good Ammonium Carbonate B-Good Ammonium Caseinate N/A Ammonium Chloride B1-Good Ammonium Hydroxide B2-Good Ammonium Nitrate B1-Good Ammonium Oxalate N/A Ammonium Persulfate D-Severe Effect Ammonium Phosphate, Dibasic B1-Good Ammonium Phosphate, Monobasic B-Good -

Download Polydrain Chemical Resistance Guide (PDF)
CHEMICAL RESISTANCE GUIDE Conventional cast-in-place trench drains are susceptible to deterioration from a wide variety of chemicals, including such common and highly present substances as salt, fuel oil, sewage, gasoline, and dilute acids. Normal concrete can absorb as much as 5% moisture. Cyclic freeze and thaw is extremely detrimental causing “break-up” and deterioration. ABT, Inc. products are designed to withstand hostile work environments and severe weather abuse. POLYDYN7 Standard ABT7, Inc. products are manufactured from PolyDyn; an advanced formulation of select quartz aggregates and inert mineral fillers bonded with high grade polyester resins. This unique material is suitable for most drainage applications, including all normal exterior and interior drainage involving exposure to salt, gasoline, fuel oil, and many dilute acids and alkalis. POLYCHAMPION7 PolyChampion is formulated to resist heavily concentrated and corrosive chemicals. Special resins of vinylester, recognized for their excellent corrosion resistance, are used as binders in the polymer concrete mixture. Only inert quartz minerals are used as aggregates. GRATINGS ABT, Inc. offers a variety of gratings that are manufactured to offer corrosion resistance. Fiberglass mesh grates formulated with vinylester resins offer corrosion resistance properties very similar to PolyChampion. Grates are supplied with stainless steel locking devices and bolts. Slotted grates and solid covers in stainless steel are also available. CHARTS This guide is intended to provide engineers and designers with specific chemical resistance information for the PolyDrain system. Chemicals, test concentrations and maximum recommended temperatures for both PolyDyn and PolyChampion are listed. An asterisk (*) indicates that no data is available. N/R denotes “Not Recommended”. -

Chemical Capabilities Listing Laboratory, R&D, Industrial and Manufacturing Applications
Page 1 of 3 Chemical Capabilities Listing Laboratory, R&D, Industrial and Manufacturing Applications Acacia, Gum Arabic Barium Oxide Chromium Trioxide Acetaldehyde Bentonite, White Citric Acid, Anhydrous Acetamide Benzaldehyde Citric Acid, Monohydrate Acetanilide Benzoic Acid Cobalt Oxide Acetic Acid Benzoyl Chloride Cobaltous Acetate Acetic Anhydride Benzyl Alcohol Cobaltous Carbonate Acetone Bismuth Chloride Cobaltous Chloride Acetonitrile Bismuth Nitrate Cobaltous Nitrate Acetyl Chloride Bismuth Trioxide Cobaltous Sulfate Aluminium Ammonium Sulfate Boric Acid Cottonseed Oil Aluminon Boric Anhydride Cupferron Aluminum Chloride, Anhydrous Brucine Sulfate Cupric Acetate Aluminum Chloride, Hexahydrate n-Butyl Acetate Cupric Bromide Aluminum Fluoride n-Butyl Alcohol Cupric Carbonate, Basic Aluminum Hydroxide tert-Butyl Alcohol Cupric Chloride Aluminum Nitrate Butyric Acid Cupric Nitrate Aluminum Oxide Cadmium Acetate Cupric Oxide Aluminum Potassium Sulfate Cadmium Carbonate Cupric Sulfate, Anhydrous Aluminum Sulfate Cadmium Chloride, Anhydrous Cupric Sulfate, Pentahydrate 1-Amino-2-Naphthol-4-Sulfonic Acid Cadmium Chloride, Hemipentahydrate Cuprous Chloride Ammonium Acetate Cadmium Iodide Cuprous Oxide, Red Ammonium Bicarbonate Cadmium Nitrate Cyclohexane Ammonium Bifluoride Cadmium Oxide Cyclohexanol Ammonium Bisulfate Cadmium Sulfate, Anhydrous Cyclohexanone Ammonium Bromide Cadmium Sulfate, Hydrate Devarda's Alloy Ammonium Carbonate Calcium Acetate Dextrose, Anhydrous Ammonium Chloride Calcium Carbide Diacetone Alcohol Ammonium Citrate