Redalyc.HISTOLOGICAL DESCRIPTION of THE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Turtles #1 Among All Species in Race to Extinction
Turtles #1 among all Species in Race to Extinction Partners in Amphibian and Reptile Conservation and Colleagues Ramp Up Awareness Efforts After Top 25+ Turtles in Trouble Report Published Washington, DC (February 24, 2011)―Partners in Amphibian and Reptile Conservation (PARC), an Top 25 Most Endangered Tortoises and inclusive partnership dedicated to the conservation of Freshwater Turtles at Extremely High Risk the herpetofauna--reptiles and amphibians--and their of Extinction habitats, is calling for more education about turtle Arranged in general and approximate conservation after the Turtle Conservation Coalition descending order of extinction risk announced this week their Top 25+ Turtles in Trouble 1. Pinta/Abingdon Island Giant Tortoise report. PARC initiated a year-long awareness 2. Red River/Yangtze Giant Softshell Turtle campaign to drive attention to the plight of turtles, now the fastest disappearing species group on the planet. 3. Yunnan Box Turtle 4. Northern River Terrapin 5. Burmese Roofed Turtle Trouble for Turtles 6. Zhou’s Box Turtle The Turtle Conservation Coalition has highlighted the 7. McCord’s Box Turtle Top 25 most endangered turtle and tortoise species 8. Yellow-headed Box Turtle every four years since 2003. This year the list included 9. Chinese Three-striped Box Turtle/Golden more species than previous years, expanding the list Coin Turtle from a Top 25 to Top 25+. According to the report, 10. Ploughshare Tortoise/Angonoka between 48 and 54% of all turtles and tortoises are 11. Burmese Star Tortoise considered threatened, an estimate confirmed by the 12. Roti Island/Timor Snake-necked Turtle Red List of the International Union for the 13. -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Cranial Cavitry
Embryology Endo, Energy, and Repro 2017-2018 EMBRYOLOGY OF THE REPRODUCTIVE SYSTEM Janine Prange-Kiel, Ph.D. Office: L1.106, Phone: 83117 Email: [email protected] LEARNING OBJECTIVES: • Name the structures in kidney development that contribute to the development of the reproductive organs. • Predict how the presence or absence of the Y chromosome and the expression of the SRY gene would influence the development of the gonads. • Predict how the presence or absence of testosterone, dihydrotestosterone, and anit- Mullerian hormone would influence the development of the genital ducts and indifferent primordia of the external genitalia. I. Introduction In general, the function of the genital (reproductive) system in males and females is the formation, nurture, and transport of germ cells. In females, an additional function is to provide the proper milieu for the fetal development after conception. Like the urinary system, the genital system derives from intermediate mesoderm. The development of these two systems is tightly interwoven as structures that develop as parts of the urinary system gain function in the genital system. In the adult, the sexual organs differ between males and females. The early genital system, however, is similar in both sexes, and the sexual differentiation of this initially indifferent, bipotential system starts only in the seventh week of embryonic development. The details on how sexual differentiation is determined will be discussed below, but it is worth mentioning here that irregularities in this process result in disorders of sexual differentiation (DSDs). DSDs occur in approximately 1 in 4,500 live births and will be discussed in a separate lecture. -

Short Communication Clinical and Pathological
Reprod Dom Anim 45, 368–372 (2010); doi: 10.1111/j.1439-0531.2009.01495.x ISSN 0936-6768 Short Communication Clinical and Pathological Findings of a Sac-like Formation in the Tunica Vaginalis of a Nelore (Bos indicus) Bull J Chaco´n1 and A Berrocal2,* 1Section of Andrology, Research Program on Applied Animal Andrology; 2Department of Pathology, School of Veterinary Medicine, Universidad, Nacional (UNA), Heredia, Costa Rica Contents and abnormalities in the ejaculate compared with A seven-month-old purebred Nelore calf was diagnosed with a normal bulls (Chaco´n et al. 1999; Chaco´n 2001). bilateral finger-shaped swelling although more prominent at Regarding the tunica vaginalis in the bull, abnormal- the left side of the scrotum, located longitudinal and parallel to ities reported in this serosa are mainly limited to epididymis corpus. The finding was present from 7 months of conditions such as hydrocele and adherences within age up to castration (performed at 25 months of age). Scrotal tunica layers secondary to orchitis. Blom and Christen- circumference, testicular and epididymis consistency and sen (1958) described also the presence of cyst like symmetry as well as seminal quality were normal during the formations (paradidymis), in the funiculus spermaticus follow-up period. The ultrasonographic appearance of the scrotal wall, pampiniform plexus, gonad and epididymis was (mesorchium), presumable derived from remnants of the normal. However, an anechoic region surrounded by a wall mesonephric duct. It is unknown that sac-like forma- forming a sac-like structure with a blind end at its dorsal pole tions in the tunica vaginalis of the bull or any other was seen on the swelling area. -

WILDLIFE TRADE in AMAZON COUNTRIES: an ANALYSIS of TRADE in CITES-LISTED SPECIES Note by the Executive Secretary 1
CBD Distr. GENERAL CBD/SBSTTA/21/INF/8 17 November 2017 ENGLISH ONLY SUBSIDIARY BODY ON SCIENTIFIC, TECHNICAL AND TECHNOLOGICAL ADVICE Twenty-first meeting Montreal, Canada, 11-14 December 2017 Item 4 of the provisional agenda* WILDLIFE TRADE IN AMAZON COUNTRIES: AN ANALYSIS OF TRADE IN CITES-LISTED SPECIES Note by the Executive Secretary 1. The Executive Secretary is circulating herewith, for the information of participants in the twenty-first meeting of the Subsidiary Body on Scientific, Technical and Technological Advice, a report presenting a comprehensive overview of international trade in wildlife species listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in the Amazon countries: Bolivia; Brazil; Colombia; Ecuador; Guyana; Peru; Suriname; and Venezuela. The analysis provides a baseline of information on trade levels and trends in these countries for the 10-year period 2005-2014, in order to inform trade management in the region. It has been produced in close collaboration with national experts, presenting contextual information and insights into the management of wildlife trade in the region. 2. The report is relevant to the work of the Convention on Biological Diversity, in particular with regard to decision XIII/8, paragraph 5(d), in which the Conference of the Parties requests the Executive Secretary, in collaboration with other members of the Collaborative Partnership on Sustainable Wildlife Management, to continue to support efforts by Parties to combat illicit trafficking in wildlife, in line with United Nations General Assembly resolution 69/314 of 30 July 2015, and to enhance institutional capacities on wildlife conservation and law enforcement with relevant law enforcement bodies, such as the International Consortium on Combating Wildlife Crime. -

ANA214: Systemic Embryology
ANA214: Systemic Embryology ISHOLA, Azeez Olakune [email protected] Anatomy Department, College of Medicine and Health Sciences Outline • Organogenesis foundation • Urogenital system • Respiratory System • Kidney • Larynx • Ureter • Trachea & Bronchi • Urinary bladder • Lungs • Male urethra • Female urethra • Cardiovascular System • Prostate • Heart • Uterus and uterine tubes • Blood vessels • Vagina • Fetal Circulation • External genitalia • Changes in Circulation at Birth • Testes • Gastrointestinal System • Ovary • Mouth • Nervous System • Pharynx • Neurulation • GI Tract • Neural crests • Liver, Spleen, Pancreas Segmentation of Mesoderm • Start by 17th day • Under the influence of notochord • Cells close to midline proliferate – PARAXIAL MESODERM • Lateral cells remain thin – LATERAL PLATE MESODERM • Somatic/Parietal mesoderm – close to amnion • Visceral/Splanchnic mesoderm – close to yolk sac • Intermediate mesoderm connects paraxial and lateral mesoderm Paraxial Mesoderm • Paraxial mesoderm organized into segments – SOMITOMERES • Occurs in craniocaudal sequence and start from occipital region • 1st developed by day 20 (3 pairs per day) – 5th week • Gives axial skeleton Intermediate Mesoderm • Differentiate into Urogenital structures • Pronephros, mesonephros Lateral Plate Mesoderm • Parietal mesoderm + ectoderm = lateral body wall folds • Dermis of skin • Bones + CT of limb + sternum • Visceral Mesoderm + endoderm = wall of gut tube • Parietal mesoderm surrounding cavity = pleura, peritoneal and pericardial cavity • Blood & -
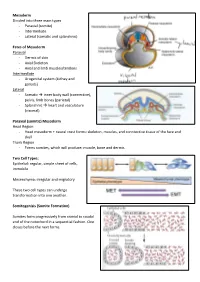
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

46/Xx Chromosome Constitution of a True Hermaphrodite
24 April 1965 S.A. TYDSKRIF VIR GENEESKUNDE 327 46/XX CHROMOSOME CONSTITUTION OF A TRUE HERMAPHRODITE S. H. FRJEDBERG, Department of Anatomy, University of the Witwarersrand Medical School, Johannesburg, AND E. E. ROSENBERG, Department of Physiology, FacullY of Medicine, University of Natal Medical School, Durban The influence of the Y-~hromosome in sex deyelopment of a normal-sized bicornuate uterus, through the inguinal is apparently considerable, since testes and a male pheno canal, into the labium majus. Here it was associated with a mass which was partially covered with a processus vaginalis type are observed in individuals who possess as many as and had the outward appearance of an ovary. This proved 5 X-chromosomes in addition to the Y.l Since a single histologically to be an ovotestis (Fig. 3). It contained numerous X-chromosome in the absence of a Y results in a female corpora lutea, a few corpora albicantia and seminiferous phenotype, it appears in fact that the Y -chromosome is a tubules with marked thickening and hyalinosis of the base ment membrane. The tubules were lined by Sertoli-like cells prerequisite for the development of human testicular and a few spermatogonia. There was no evidence of spermato tissue. The fact that the majority of true hermaphrodites genesis. The interstitial cells of Leydig were hyperplastic (Fig. (19 out of 25) have a normal female XX complex2 is thus 4). puzzling. It would seem that though the Y-chromosome The left gonad was cystic and haemorrhagic and proved histologically to consist only of ovarian tissue. The left has considerable influence in testicular differentiation, it uterine tube had undergone torsion. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -

1- Development of Female Genital System
Development of female genital systems Reproductive block …………………………………………………………………. Objectives : ✓ Describe the development of gonads (indifferent& different stages) ✓ Describe the development of the female gonad (ovary). ✓ Describe the development of the internal genital organs (uterine tubes, uterus & vagina). ✓ Describe the development of the external genitalia. ✓ List the main congenital anomalies. Resources : ✓ 435 embryology (males & females) lectures. ✓ BRS embryology Book. ✓ The Developing Human Clinically Oriented Embryology book. Color Index: ✓ EXTRA ✓ Important ✓ Day, Week, Month Team leaders : Afnan AlMalki & Helmi M AlSwerki. Helpful video Focus on female genital system INTRODUCTION Sex Determination - Chromosomal and genetic sex is established at fertilization and depends upon the presence of Y or X chromosome of the sperm. - Development of female phenotype requires two X chromosomes. - The type of sex chromosomes complex established at fertilization determine the type of gonad differentiated from the indifferent gonad - The Y chromosome has testis determining factor (TDF) testis determining factor. One of the important result of fertilization is sex determination. - The primary female sexual differentiation is determined by the presence of the X chromosome , and the absence of Y chromosome and does not depend on hormonal effect. - The type of gonad determines the type of sexual differentiation in the Sexual Ducts and External Genitalia. - The Female reproductive system development comprises of : Gonad (Ovary) , Genital Ducts ( Both male and female embryo have two pair of genital ducts , They do not depend on ovaries or hormones ) and External genitalia. DEVELOPMENT OF THE GONADS (ovaries) - Is Derived From Three Sources (Male Slides) 1. Mesothelium 2. Mesenchyme 3. Primordial Germ cells (mesodermal epithelium ) lining underlying embryonic appear among the Endodermal the posterior abdominal wall connective tissue cell s in the wall of the yolk sac). -

Setting the Stage for Understanding Globalization of the Asian Turtle Trade
Setting the Stage for Understanding Globalization of the Asian Turtle Trade: Global, Asian, and American Turtle Diversity, Richness, Endemism, and IUCN Red List Threat Levels Anders G.J. Rhodin and Peter Paul van Dijk IUCN Tortoise and Freshwater Turtle Specialist Group, Chelonian Research Foundation, Conservation International Thursday, January 20, 2011 New Species Described 2010 Photo C. Hagen Graptemys pearlensis - Pearl River Map Turtle Louisiana and Mississippi, USA Red List: Not Evaluated [Endangered] Thursday, January 20, 2011 IUCN/SSC Tortoise and Freshwater Turtle Specialist Group Founded 1980 www.iucn-tftsg.org Thursday, January 20, 2011 International Union for the Conservation of Nature / Species Survival Commission www.iucn.org Thursday, January 20, 2011 Convention on International Trade in Endangered Species of Fauna and Flora www.cites.org Thursday, January 20, 2011 Chelonian Conservation and Biology Thomson Reuters’ ISI Journal Citation Impact Factor currently ranks CCB among the top 100 zoology journals worldwide www.chelonianjournals.org Thursday, January 20, 2011 Conservation Biology of Freshwater Turtles and Tortoises www.iucn-tftsg.org/cbftt Thursday, January 20, 2011 IUCN Tortoise and Freshwater Turtle Specialist Group Members: Work or Focus - 2010 274 Members - 107 Countries Thursday, January 20, 2011 Species, Additional Subspecies, and Total Taxa of Turtles and Tortoises 500 Species Add. Subspecies 375 Total Taxa 250 125 0 1758176617831789179218011812183518441856187318891909193419551961196719771979198619891992199420062007200820092010 Currently Recognized: 334 species, 127 add. subspecies, 461 total taxa Thursday, January 20, 2011 Tortoise and Freshwater Turtle Species Richness Buhlmann, Akre, Iverson, Karapatakis, Mittermeier, Georges, Rhodin, van Dijk, and Gibbons. 2009. Chelonian Conservation and Biology 8:116–149. Thursday, January 20, 2011 Tortoise and Freshwater Turtle Species Richness – Global Rankings 1.