MRI Brain White Matter Change: Spectrum of Change – How Can We Grade? K Forbes1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’S Disease
life Review The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease Gianfranco Natale 1,2,* , Larisa Ryskalin 1 , Gabriele Morucci 1 , Gloria Lazzeri 1, Alessandro Frati 3,4 and Francesco Fornai 1,4 1 Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, 56126 Pisa, Italy; [email protected] (L.R.); [email protected] (G.M.); [email protected] (G.L.); [email protected] (F.F.) 2 Museum of Human Anatomy “Filippo Civinini”, University of Pisa, 56126 Pisa, Italy 3 Neurosurgery Division, Human Neurosciences Department, Sapienza University of Rome, 00135 Rome, Italy; [email protected] 4 Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.) Neuromed, 86077 Pozzilli, Italy * Correspondence: [email protected] Abstract: The gastrointestinal (GI) tract is provided with a peculiar nervous network, known as the enteric nervous system (ENS), which is dedicated to the fine control of digestive functions. This forms a complex network, which includes several types of neurons, as well as glial cells. Despite extensive studies, a comprehensive classification of these neurons is still lacking. The complexity of ENS is magnified by a multiple control of the central nervous system, and bidirectional communication between various central nervous areas and the gut occurs. This lends substance to the complexity of the microbiota–gut–brain axis, which represents the network governing homeostasis through nervous, endocrine, immune, and metabolic pathways. The present manuscript is dedicated to Citation: Natale, G.; Ryskalin, L.; identifying various neuronal cytotypes belonging to ENS in baseline conditions. -

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

The Enteric Nervous System: a Second Brain
The Enteric Nervous System: A Second Brain MICHAEL D. GERSHON Columbia University Once dismissed as a simple collection of relay ganglia, the enteric nervous system is now recognized as a complex, integrative brain in its own right. Although we still are unable to relate complex behaviors such as gut motility and secretion to the activity of individual neurons, work in that area is proceeding briskly--and will lead to rapid advances in the management of functional bowel disease. Dr. Gershon is Professor and Chair, Department of Anatomy and Cell Biology, Columbia University College of Physicians and Surgeons, New York. In addition to numerous scientific publications, he is the author of The Second Brain (Harper Collins, New York, 1998). Structurally and neurochemically, the enteric nervous system (ENS) is a brain unto itself. Within those yards of tubing lies a complex web of microcircuitry driven by more neurotransmitters and neuromodulators than can be found anywhere else in the peripheral nervous system. These allow the ENS to perform many of its tasks in the absence of central nervous system (CNS) control--a unique endowment that has permitted enteric neurobiologists to investigate nerve cell ontogeny and chemical mediation of reflex behavior in a laboratory setting. Recognition of the importance of this work as a basis for developing effective therapies for functional bowel disease, coupled with the recent, unexpected discovery of major enteric defects following the knockout of murine genes not previously known to affect the gut, has produced a groundswell of interest that has attracted some of the best investigators to the field. Add to this that the ENS provides the closest thing we have to a window on the brain, and one begins to understand why the bowel--the second brain--is finally receiving the attention it deserves. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

The Remarkable, Yet Not Extraordinary, Human Brain As a Scaled-Up Primate Brain and Its Associated Cost
The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost Suzana Herculano-Houzel1 Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, 21941-902, Rio de Janeiro, Brazil; and Instituto Nacional de Neurociência Translacional, Instituto Nacional de Ciência e Tecnologia/Ministério de Ciência e Tecnologia, 04023-900, Sao Paulo, Brazil Edited by Francisco J. Ayala, University of California, Irvine, CA, and approved April 12, 2012 (received for review February 29, 2012) Neuroscientists have become used to a number of “facts” about the The incongruity between our extraordinary cognitive abilities human brain: It has 100 billion neurons and 10- to 50-fold more glial and our not-that-extraordinary brain size has been the major cells; it is the largest-than-expected for its body among primates driving factor behind the idea that the human brain is an outlier, and mammals in general, and therefore the most cognitively able; an exception to the rules that have applied to the evolution of all it consumes an outstanding 20% of the total body energy budget other animals and brains. A largely accepted alternative expla- despite representing only 2% of body mass because of an increased nation for our cognitive superiority over other mammals has been metabolic need of its neurons; and it is endowed with an overde- our extraordinary brain size compared with our body size, that is, veloped cerebral cortex, the largest compared with brain size. our large encephalization quotient (8). Compared -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -
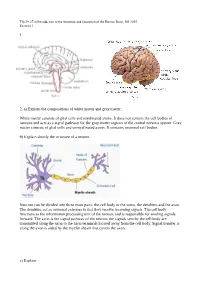
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

White Matter Hyperintensity Accumulation During Treatment of Late-Life Depression
Neuropsychopharmacology (2015) 40, 3027–3035 © 2015 American College of Neuropsychopharmacology. All rights reserved 0893-133X/15 www.neuropsychopharmacology.org White Matter Hyperintensity Accumulation During Treatment of Late-Life Depression 1 1 1 1 1 Alexander Khalaf , Kathryn Edelman , Dana Tudorascu , Carmen Andreescu , Charles F Reynolds and ,1 Howard Aizenstein* 1 Western Psychiatric Institute and Clinic, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA White matter hyperintensities (WMHs) have been shown to be associated with the development of late-life depression (LLD) and eventual treatment outcomes. This study sought to investigate longitudinal WMH changes in patients with LLD during a 12-week antidepressant treatment course. Forty-seven depressed elderly patients were included in this analysis. All depressed subjects started pharmacological treatment for depression shortly after a baseline magnetic resonance imaging (MRI) scan. At 12 weeks, patients = = underwent a follow-up MRI scan, and were categorized as either treatment remitters (n 23) or non-remitters (n 24). Among all patients, there was as a significant increase in WMHs over 12 weeks (t(46) = 2.36, P = 0.02). When patients were stratified by remission status, non-remitters demonstrated a significant increase in WMHs (t(23) = 2.17, P = 0.04), but this was not observed in remitters (t(22) = 1.09, P = 0.29). Other markers of brain integrity were also investigated including whole brain gray matter volume, hippocampal volume, and fractional anisotropy. No significant differences were observed in any of these markers during treatment, including when patients were stratified based on remission status. These results add to existing literature showing the association between WMH accumulation and LLD treatment outcomes. -

Association Between Carotid Hemodynamics and Asymptomatic White and Gray Matter Lesions in Patients with Essential Hypertension
797 Hypertens Res Vol.28 (2005) No.10 p.797-803 Original Article Association between Carotid Hemodynamics and Asymptomatic White and Gray Matter Lesions in Patients with Essential Hypertension Mie KURATA, Takafumi OKURA, Sanae WATANABE, and Jitsuo HIGAKI The aim of this study was to clarify the magnitude of common carotid artery (CCA) structural and hemody- namic parameters on brain white and gray matter lesions in patients with essential hypertension (EHT). The study subjects were 49 EHT patients without a history of previous myocardial infarction, atrial fibrillation, diabetes mellitus, impaired glucose tolerance, chronic renal failure, symptomatic cerebrovascular events, or asymptomatic carotid artery stenosis. All patients underwent brain MRI and ultrasound imaging of the CCA. MRI findings were evaluated by periventricular hyperintensity (PVH), deep and subcortical white matter hyperintensity (DSWMH), and état criblé according to the Japanese Brain dock Guidelines of 2003. Intima media thickness (IMT), and mean diastolic (Vd) and systolic (Vs) velocities were evaluated by carotid ultra- sound. The Vd/Vs ratio was further calculated as a relative diastolic flow velocity. The mean IMT and max IMT were positively associated with PVH, DSWMH, and état criblé (mean IMT: = 0.473, 0.465, 0.494, p=0.0007, 0.0014, 0.0008, respectively; max IMT: = 0.558, 0.443, 0.514, p=0.0001, 0.0024, 0.0004, respec- tively). Vd/Vs was negatively associated with état criblé ( = - 0.418, p=0.0038). Carotid structure and hemo- dynamics are potentially related to asymptomatic lesions in the cerebrum, and might be predictors of future cerebral vascular events in patients with EHT. -

Neuroscience: Systems, Behavior & Plasticity 1
Neuroscience: Systems, Behavior & Plasticity 1 Neuroscience: Systems, Behavior & Plasticity Debra Bangasser, Director 873 Weiss Hall 215-204-1015 [email protected] Rebecca Brotschul, Program Coordinator 618 Weiss Hall 215-204-3441 [email protected] https://liberalarts.temple.edu/departments-and-programs/neuroscience/ A major in Neuroscience enables students to pursue a curriculum in several departments, colleges, and schools at Temple University in one of the most dynamic areas of science. Neuroscience is an interdisciplinary field addressing neural and brain function at multiple levels. It encompasses a broad domain that ranges from molecular genetics and neural development, to brain processes involved in cognition and emotion, to mechanisms and consequences of neurodegenerative disease. The field of neuroscience also includes mathematical and physical principles involved in modeling neural systems and in brain imaging. The undergraduate, interdisciplinary Neuroscience Major will culminate in a Bachelor of Science degree. Many high-level career options within and outside of the field of neuroscience are open to students with this major. This is a popular major with students aiming for professional careers in the health sciences such as in medicine, dentistry, pharmacy, physical and occupational therapy, and veterinary science. Students interested in graduate school in biology, chemistry, communications science, neuroscience, or psychology are also likely to find the Neuroscience Major attractive. Neuroscience Accelerated +1 Bachelor of Science / Master of Science Program The accelerated +1 Bachelor of Science / Master of Science in Neuroscience: Systems, Behavior and Plasticity program offers outstanding Temple University Neuroscience majors the opportunity to earn both the BS and MS in Neuroscience in just 5 years.