Colestid/Colestid Orange Granules & Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Your Patient Have Bile Acid Malabsorption?
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 Carol Rees Parrish, MS, RDN, Series Editor Does Your Patient Have Bile Acid Malabsorption? John K. DiBaise Bile acid malabsorption is a common but underrecognized cause of chronic watery diarrhea, resulting in an incorrect diagnosis in many patients and interfering and delaying proper treatment. In this review, the synthesis, enterohepatic circulation, and function of bile acids are briefly reviewed followed by a discussion of bile acid malabsorption. Diagnostic and treatment options are also provided. INTRODUCTION n 1967, diarrhea caused by bile acids was We will first describe bile acid synthesis and first recognized and described as cholerhetic enterohepatic circulation, followed by a discussion (‘promoting bile secretion by the liver’) of disorders causing bile acid malabsorption I 1 enteropathy. Despite more than 50 years since (BAM) including their diagnosis and treatment. the initial report, bile acid diarrhea remains an underrecognized and underappreciated cause of Bile Acid Synthesis chronic diarrhea. One report found that only 6% Bile acids are produced in the liver as end products of of British gastroenterologists investigate for bile cholesterol metabolism. Bile acid synthesis occurs acid malabsorption (BAM) as part of the first-line by two pathways: the classical (neutral) pathway testing in patients with chronic diarrhea, while 61% via microsomal cholesterol 7α-hydroxylase consider the diagnosis only in selected patients (CYP7A1), or the alternative (acidic) pathway via or not at all.2 As a consequence, many patients mitochondrial sterol 27-hydroxylase (CYP27A1). are diagnosed with other causes of diarrhea or The classical pathway, which is responsible for are considered to have irritable bowel syndrome 90-95% of bile acid synthesis in humans, begins (IBS) or functional diarrhea by exclusion, thereby with 7α-hydroxylation of cholesterol catalyzed interfering with and delaying proper treatment. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -
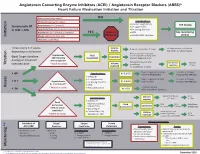
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

The Necessity and Effectiveness of Mineralocorticoid Receptor Antagonist in the Treatment of Diabetic Nephropathy
Hypertension Research (2015) 38, 367–374 & 2015 The Japanese Society of Hypertension All rights reserved 0916-9636/15 www.nature.com/hr REVIEW The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy Atsuhisa Sato Diabetes mellitus is a major cause of chronic kidney disease (CKD), and diabetic nephropathy is the most common primary disease necessitating dialysis treatment in the world including Japan. Major guidelines for treatment of hypertension in Japan, the United States and Europe recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which suppress the renin-angiotensin system (RAS), as the antihypertensive drugs of first choice in patients with coexisting diabetes. However, even with the administration of RAS inhibitors, failure to achieve adequate anti-albuminuric, renoprotective effects and a reduction in cardiovascular events has also been reported. Inadequate blockade of aldosterone may be one of the reasons why long-term administration of RAS inhibitors may not be sufficiently effective in patients with diabetic nephropathy. This review focuses on treatment in diabetic nephropathy and discusses the significance of aldosterone blockade. In pre-nephropathy without overt nephropathy, a mineralocorticoid receptor antagonist can be used to enhance the blood pressure-lowering effects of RAS inhibitors, improve insulin resistance and prevent clinical progression of nephropathy. In CKD categories A2 and A3, the addition of a mineralocorticoid receptor antagonist to an RAS inhibitor can help to maintain ‘long-term’ antiproteinuric and anti-albuminuric effects. However, in category G3a and higher, sufficient attention must be paid to hyperkalemia. Mineralocorticoid receptor antagonists are not currently recommended as standard treatment in diabetic nephropathy. -

Optimal Diuretic Strategies in Heart Failure
517 Review Article on Heart Failure Update and Advances in 2021 Page 1 of 8 Optimal diuretic strategies in heart failure Sarabjeet S. Suri, Salpy V. Pamboukian Division of Cardiovascular Diseases, University of Alabama at Birmingham, Birmingham, AL, USA Contributions: (I) Conception and design: SS Suri; (II) Administrative support: SV Pamboukian; (III) Provision of study materials or patients: Both authors; (IV) Collection and assembly of data: Both authors; (V) Data analysis and interpretation: Both authors; (VI) Manuscript writing: Both authors; (VII) Final approval of manuscript: Both authors. Correspondence to: Dr. Sarabjeet S. Suri, MD. University of Alabama at Birmingham, 1900 University Blvd, THT 321, Birmingham, AL 35223, USA. Email: [email protected]. Abstract: Heart failure (HF) is one of the major causes of morbidity and mortality in the world. According to a 2019 American Heart Association report, about 6.2 million American adults had HF between 2013 and 2016, being responsible for almost 1 million admissions. As the population ages, the prevalence of HF is anticipated to increase, with 8 million Americans projected to have HF by 2030, posing a significant public health and financial burden. Acute decompensated HF (ADHF) is a syndrome characterized by volume overload and inadequate cardiac output associated with symptoms including some combination of exertional shortness of breath, orthopnea, paroxysmal nocturnal dyspnea (PND), fatigue, tissue congestion (e.g., peripheral edema) and decreased mentation. The pathology is characterized by hemodynamic abnormalities that result in autonomic imbalance with an increase in sympathetic activity, withdrawal of vagal activity and neurohormonal activation (NA) resulting in increased plasma volume in the setting of decreased sodium excretion, increased water retention and in turn an elevation of filling pressures. -

Atorvastatin Calcium)
PRODUCT INFORMATION LIPITOR® (atorvastatin calcium) NAME OF THE MEDICINE LIPITOR atorvastatin (as calcium) 10 mg, 20 mg, 40 mg and 80 mg tablets. LIPITOR contains the active ingredient atorvastatin calcium. The structural formula of atorvastatin calcium is shown below: CH3 CH3 CH OH OH O O CH CH CH C NHC 2 - N CH2 CH2 CH2 O •Ca 2+ •3H2 O F 2 Chemical name: [R-(R*,R*)]-2-(4-fluorophenyl)-ß,-dihydroxy-5-(1-methylethyl)- 3-phenyl-4-[(phenylamino) carbonyl] -1H-pyrrole -1-heptanoic acid, calcium salt (2:1) Molecular formula: (C33H34FN2O5)2Ca.3H2O Molecular weight: 1209.42 CAS registry number: 134523-03-8. DESCRIPTION Atorvastatin calcium is a white to off-white crystalline powder that is practically insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium is very slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile, slightly soluble in ethanol and freely soluble in methanol. LIPITOR tablets contain atorvastatin calcium equivalent to 10, 20, 40 and 80 mg atorvastatin. The tablets also contain the following inactive ingredients: calcium carbonate, microcrystalline cellulose, lactose, croscarmellose sodium, polysorbate 80, hydroxypropylcellulose, magnesium stearate, Opadry White YS-1-7040 and Simethicone Emulsion. Version: pfplipit10613 Supersedes: pfplipit10512 Page 1 of 20 PHARMACOLOGY Mechanism of Action Atorvastatin is a synthetic lipid-lowering agent. Atorvastatin is an inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methyl-glutaryl-coenzyme A to mevalonate, a precursor of sterols, including cholesterol. Triglycerides (TG) and cholesterol in the liver are incorporated into very low density lipoprotein (VLDL) and released into the plasma for delivery to peripheral tissues. -

Anatomical Classification Guidelines V2020 EPHMRA ANATOMICAL
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2020 Anatomical Classification Guidelines V2020 "The Anatomical Classification of Pharmaceutical Products has been developed and maintained by the European Pharmaceutical Marketing Research Association (EphMRA) and is therefore the intellectual property of this Association. EphMRA's Classification Committee prepares the guidelines for this classification system and takes care for new entries, changes and improvements in consultation with the product's manufacturer. The contents of the Anatomical Classification of Pharmaceutical Products remain the copyright to EphMRA. Permission for use need not be sought and no fee is required. We would appreciate, however, the acknowledgement of EphMRA Copyright in publications etc. Users of this classification system should keep in mind that Pharmaceutical markets can be segmented according to numerous criteria." © EphMRA 2020 Anatomical Classification Guidelines V2020 CONTENTS PAGE INTRODUCTION A ALIMENTARY TRACT AND METABOLISM 1 B BLOOD AND BLOOD FORMING ORGANS 28 C CARDIOVASCULAR SYSTEM 35 D DERMATOLOGICALS 50 G GENITO-URINARY SYSTEM AND SEX HORMONES 57 H SYSTEMIC HORMONAL PREPARATIONS (EXCLUDING SEX HORMONES) 65 J GENERAL ANTI-INFECTIVES SYSTEMIC 69 K HOSPITAL SOLUTIONS 84 L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS 92 M MUSCULO-SKELETAL SYSTEM 102 N NERVOUS SYSTEM 107 P PARASITOLOGY 118 R RESPIRATORY SYSTEM 120 S SENSORY ORGANS 132 T DIAGNOSTIC AGENTS 139 V VARIOUS 141 Anatomical Classification Guidelines V2020 INTRODUCTION The Anatomical Classification was initiated in 1971 by EphMRA. It has been developed jointly by Intellus/PBIRG and EphMRA. It is a subjective method of grouping certain pharmaceutical products and does not represent any particular market, as would be the case with any other classification system. -

Combination Therapy
Combination Antihypertensive Therapy: When to use it Diabetes George L. Bakris, MD, F.A.S.N., F.A.S.H. Professor of Medicine Director, ASH Comprehensive Hypertension Center The University of Chicago Medicine Development of Antihypertensive Therapies ? More Effective but As effective and As effective and even effective for poorly tolerated better tolerated better tolerated SBP 1940s 1950 1957 1960s 1970s 1980s 1990s 2001- 2009 ARBs Direct Direct ACE inhibitors Renin vasodilators inhibitors α-blockers Peripheral Thiazides ETa sympatholytics diuretics Blockers Central ααα 2 Calcium Ganglion agonists blockers antagonists- VPIs Calcium DHPs Others Veratrum antagonists- alkaloids non DHPs βββ-blockers Evolution of Fixed Dose Combination Antihypertensive Therapies 1960s 1970s 1980s 1990s 2000- present Combination CCBs+ ARBs Diuretics RAS Blockers ARB + Aldactazide, with CCBs chlorthalidone Dyazide, Maxzide, DRIs +ARBs Guanabenz SerApAs RAS Blockers (Lotrel) DRIs+ CCBs (reserpine, with diuretics hydralazine, Beta blocker +diuretics TRIPLE Combos HCTZ) (CCB+RAS Blocker + diuretic) Rationale for Fixed-Dose Combination Therapy: Background • Traditional antihypertensive therapy yields goal BP in <60% of treated hypertensive patients1-3 • Switching from one monotherapy to another is effective in only about 50% of patients1 • Most patients will require at least two drugs to attain goal BP (<140/90 mm Hg, or <130/80 mm Hg for patients with diabetes or chronic renal disease)4-6 BP = blood pressure 1. Materson BJ et al. J Hum Hypertens. 1995;9(10):791-796. 2. Messerli FH. J Hum Hypertens. 1992;6 Suppl. 2:S19-S21. 3. Ram CV. J Clin Hypertens (Greenwich). 2004;6(10):569-577. 4. Chobanian AV, et al. JAMA. -

The Effect of Colestipol on Glycemic Control in Patients with Type 2 Diabetes
The Effect of Colestipol on Glycemic Control in Patients With Type 2 Diabetes Elizabeth A. Sauter, PharmD; Sindhu Abraham, PharmD, BCPS; Tania G. John, PharmD; Seema Kapadia, PharmD, BCACP; and Judith A. Toth, PharmD, CGP, CDE Using an electronic chart review, the authors investigated the effect of colestipol, a bile acid sequestrant, on glycemic control in patients with type 2 diabetes. iabetes mellitus is a complex cations. In general, every percentage group and the placebo group of disease, characterized by hy- point drop in glycosylated hemoglo- − 0.5% (P = .007). Additionally, in perglycemia, and can be as- bin (A1C) can reduce the risk of mi- participants with a baseline A1C ≥ sociated with abnormalities crovascular complications (ie, eye, 8%, the difference in mean change in D 2 in fat, carbohydrates, and protein kidney, and nerve diseases) by 40%. A1C was − 1.0% (P = .002). Treat- metabolism that result from defects Improved control of LDL-C can re- ment with colesevelam was also in insulin secretion, insulin action, duce cardiovascular complications by associated with a reduction in post- or both. Type 2 diabetes is the most 20% to 50%.2 Current American Dia- prandial glucose (− 31.5 mg/dL, common form of diabetes in the betes Association (ADA) guidelines P = .026) and LDL-C (− 11.7%, United States, accounting for as many recommend an A1C of < 7% and an P = .007). The study authors con- as 90% to 95% of all cases.1 As of LDL-C < 100 mg/dL in patients with cluded that colesevelam may improve 2011, 25.8 million patients were di- diabetes.4 However, to achieve these both glycemic and lipid control in agnosed with diabetes, or 8.3% of the goals, patients with diabetes often patients with type 2 diabetes.5 Fol- U.S.