Mistaken Identity? Invasive Plants and Their Native Look-Alikes: an Identification Guide for the Mid-Atlantic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Coptis Trifolia Conservation Assessment
CONSERVATION ASSESSMENT for Coptis trifolia (L.) Salisb. Originally issued as Management Recommendations December 1998 Marty Stein Reconfigured-January 2005 Tracy L. Fuentes USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington CONSERVATION ASSESSMENT FOR COPTIS TRIFOLIA Table of Contents Page List of Tables ................................................................................................................................. 2 List of Figures ................................................................................................................................ 2 Summary........................................................................................................................................ 4 I. NATURAL HISTORY............................................................................................................. 6 A. Taxonomy and Nomenclature.......................................................................................... 6 B. Species Description ........................................................................................................... 6 1. Morphology ................................................................................................................... 6 2. Reproductive Biology.................................................................................................... 7 3. Ecological Roles ............................................................................................................. 7 C. Range and Sites -

2014 Plant Species List
Acanthaceae Hygrophila Occasional lacustris Acanthaceae Justicia ovata Uncommon Acanthaceae Ruellia humilis Common Acanthaceae Ruellia nudiflora s.n. Uncommon Acanthaceae Ruellia Occasional pedunculata Aceraceae Acer rubrum Occasional Agavaceae Yucca louisianica Uncommon Aiozaceae Molluga Occasional verticillata Alismataceae Echinodorus Occasional cordifolius Alismataceae Sagittaria Rare papillosa Alismataceae Sagittaria 156 Uncommon platyphylla Alliaceae Allium Occasional canadense var. canadense Alliaceae Allium Occasional canadense var. mobilense Alliaceae Allium 96, Uncommon drummondii 124 (Keith 96, 124) Amaranthaceae Alternanthera Common philoxeroides Amaryllidaceae Hymenocallis Uncommon liriosome Anacardiaceae Rhus aromatica Uncommon Anacardiaceae Rhus copallinum Occasional Anacardiaceae Toxicodendron Frequent radicans Apiaceae Bifora americana Common Apiaceae Centella erecta Uncommon Apiaceae Chaerophyllum Uncommon tainturieri Apiaceae Cicuta Uncommon maculatum Apiaceae Cynosciadium Uncommon digitatum Apiaceae Eryngium Common yuccifolium Apiaceae Hydrocotyle Occasional verticillata Apiaceae Polytaenia Frequent texana Apiaceae Ptilimnium Common capillaceum Apiaceae Ptilimnium Common nuttallii Apiaceae Spermolepsis Common inermis Apiaceae Torilis arvensis Occasional Apocynaceae Apocynum Occasional cannibinum Apocynaceae Nerium oleander Rare Apocynaceae Trachelospermu Occasional m difforme Aquifoliaceae Ilex decidua Common Aquifoliaceae Ilex opaca Common Aquifoliaceae Ilex vomitoria Abundant Araceae Arisaema Rare dracontium Araceae -

Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Spring 5-2016 Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi Hanna M. Miller University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Biodiversity Commons, and the Botany Commons Recommended Citation Miller, Hanna M., "Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi" (2016). Honors Theses. 389. https://aquila.usm.edu/honors_theses/389 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi by Hanna Miller A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirement for the Degree of Bachelor of Science in the Department of Biological Sciences May 2016 ii Approved by _________________________________ Mac H. Alford, Ph.D., Thesis Adviser Professor of Biological Sciences _________________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences _________________________________ Ellen Weinauer, Ph.D., Dean Honors College iii Abstract The North American Coastal Plain contains some of the highest plant diversity in the temperate world. However, most of the region has remained unstudied, resulting in a lack of knowledge about the unique plant communities present there. -
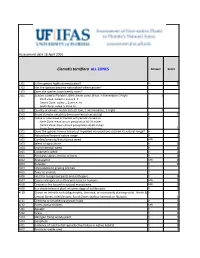
WRA.Datasheet.Template (Version 1) (Version 1).Xlsx
Assessment date 18 April 2016 Clematis terniflora ALL ZONES Answer Score 1.01 Is the species highly domesticated? n 0 1.02 Has the species become naturalised where grown? 1.03 Does the species have weedy races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality of climate match data (0-low; 1-intermediate; 2-high) 2 2.03 Broad climate suitability (environmental versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation y North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 1 2.05 Does the species have a history of repeated introductions outside its natural range? y 3.01 Naturalized beyond native range y 2 3.02 Garden/amenity/disturbance weed unk 3.03 Weed of agriculture n 0 3.04 Environmental weed y 4 3.05 Congeneric weed y 2 4.01 Produces spines, thorns or burrs n 0 4.02 Allelopathic unk 0 4.03 Parasitic n 0 4.04 Unpalatable to grazing animals ? 4.05 Toxic to animals ? 4.06 Host for recognised pests and pathogens n 0 4.07 Causes allergies or is otherwise toxic to humans unk 0 4.08 Creates a fire hazard in natural ecosystems unk 0 4.09 Is a shade tolerant plant at some stage of its life cycle n 0 4.10 Grows on infertile soils (oligotrophic, limerock, or excessively draining soils). -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

MANAGING the TOP FIVE INVASIVE PLANTS in CALVERT COUNTY 1. What Are the Top 5 Invasive Plants in Calvert County? Phragmites
MANAGING THE TOP FIVE INVASIVE PLANTS IN CALVERT COUNTY 1. What are the top 5 invasive plants in Calvert County? Phragmites, English Ivy, Kudzu, Bamboo and Autumn Olive. Phragmites common reed (Phragmites australis) Accidentally imported in ship ballast, European phragmites has spread up and down the East Coast for centuries, displacing millions of acres of wetland plants, including native phragmites. The Maryland Eastern Shore may harbor our only remaining native phragmites (Phragmites australis ssp. americanus.) Whereas native phragmites grows in sparse clumps and decomposes readily, European phragmites forms impenetrable monocultures more than 15-feet tall composed of both old and new canes. Subsequently, infested wetlands become dry land, wildlife habitat is destroyed, and a fire hazard created. Roots penetrate several feet deep and extend out 10 feet a season. Wind and water, carrying seed and rhizome/root fragments, have spread phragmites to tidal and nontidal wetland and dry lands, including ditches. It is extremely difficult to combat. English ivy (Hedera helix) Eurasian, not English, in origin, this evergreen vine threatens habitats at all heights. At ground level, its leaves shade out seedlings and herbs, forming acres of monoculture and attracting rodents. In trees, it engulfs branches, shading and slowly killing them. Its weight topples trees in wind, snow or icy conditions. It serves as a reservoir for bacterial leaf scorch, a serious disease of trees including maples, oaks and elms. Vines mature in trees, then flower and bear toxic berries which induce birds to vomit them out, ensuring spread. Any rooted piece can resprout. Kudzu (Pueraria montana var. lobata) “The vine that ate the South” was promoted as livestock forage, an ornamental, and for erosion control until the 1950s. -

Miscanthus Sinensis
As a neighbor or property owner, you Resources for more information: can play a special role in protecting the Websites Conservancy. How can Wisconsin DNR’s website, www.dnr.wi.us. This brochure covers seven invasive plants that Recommended search terms: Euonymus, Common buckthorn, Eurasian bush honeysuckle land managers are targeting for control in the neighbors Conservancy. Wisconsin First Detector Network (WIFDN), fyi.extension.wisc.edu/wifdn. On the right side, click help protect the Invasive plants pose a threat to the Pheasant on “Access fact sheets and ID videos” and scroll down Branch Conservancy. to Terrestrial Plants. Get fact sheets for Bird’s-foot trefoil, Buckthorns, Bush honeysuckles/Japanese Pheasant Branch honeysuckle, Crown vetch Invasive plants can disrupt or degrade diversity and function of an ecosystem resulting in The Invasive Plant Atlas of the United States, Conservancy? simplified and less resiliant plant communities. invasiveplantatlas.org. Click on the tabs (Grasses, Herbs/Forbs, Shrubs/Subshrubs). The plants are arranged alphabetically by scientific name. Click on While land managers and volunteers spend the plant name for detailed information. time and resources to remove invasive plants Grasses – Miscanthus sinensis from the Conservancy, their efforts can be Herbs/Forbs – Lotus corniculatus, Securigera varia hampered by neighboring properties that Shrubs/Subshrubs – Euonymus alatus, Euonymus harbor the very species they are targeting. europaeus, Lonicera (various species), Rhamnus cathartica Invasive plants can easily move across the land- Invasive Plants Association of Wisconsin,www.ipaw. scape. Birds and other animals eat seeds and org. deposit them in their droppings. Wind carries fluffy and winged seeds great distances. -

Araliaceae – Ginseng Family
ARALIACEAE – GINSENG FAMILY Plant: some herbs (perennial), woody vines, shrubs and trees Stem: usually pithy Root: sometimes with rhizomes Leaves: simple or palmately compound but rarely 2’s or 3’s, often thickened and large, mostly alternate (rarely opposite or whorled); usually with stipules that forms a stem sheath; often with star-shaped hairs Flowers: mostly perfect or unisexual (monoecious or dioecious), regular (actinomorphic); flowers very small, mostly in umbels; sepals 5, often forming small teeth or none, mostly 5(-10) petals; mostly 5(-10) stamens; ovary inferior, 2-5 (10) fused carpels Fruit: berry or drupe, oily Other: mostly tropical and subtropical, a few oranamentals; similar to Apiaceae; Dicotyledons Group Genera: 70+ genera; locally Aralia (spikenard), Hedera (English Ivy), Oplopanax, Panax (ginseng) WARNING – family descriptions are only a layman’s guide and should not be used as definitive Araliaceae (Ginseng Family) – 5 (mostly) sepals and petals (often 5-lobed), often in umbels or compound umbels; leaves simple or more often compound; fruit a berry or drupe Examples of common genera Devil's Walkingstick [Hercules’ Club] Wild Sarsaparilla Aralia spinosa L. Aralia nudicaulis L. Devil's Club [Devil’s Walking Stick; Alaskan Ginseng] Oplopanax horridus (Sm.) Miq. English Ivy Hedera helix L. (Introduced) Dwarf Ginseng Panax trifolius L. ARALIACEAE – GINSENG FAMILY Wild Sarsaparilla; Aralia nudicaulis L. Devil's Walkingstick [Hercules’ Club]; Aralia spinosa L. English Ivy; Hedera helix L. (Introduced) Devil's Club [Devil’s -

Studies of Fruit and Seed Characters of Selected Euonymus Species Bernd Schulz, Translated by Wolfgang Bopp
Studies of fruit and seed characters of selected Euonymus species Bernd Schulz, translated by Wolfgang Bopp Summary Euonymus species are particularly prized for their decorative fruits. This work describes and illustrates (in water colour), the fruits and seeds of 30 commonly cultivated species found in Central European plant collections. The text discusses the criteria for species differentiation. As well as the shape of the fruits and seeds, seed colouration is described and compared to several published accounts in an attempt to correct previous misconceptions. This paper was originally published in German in 2006 (Schulz, 2006a). This translation contains some minor additions and adjustments to the original text and has been translated to the best of our ability, albeit not being a 100% accurate translation. 1 Introduction The German common name of Euonymus europaeus, is “Pfaffenhütchen” which translates as priest’s hat or mitre, in reference to its shape. In past centuries, the wearing of such a hat was a status symbol, showing the belonging to a professional group. Priests in Germany have, since the sixteenth century, been wearing the so-called biretta1,”a stiff and square-shaped head covering as worn by priests, also found in a wider and bigger version“ (Loschek 1993). In Germany today certain clerics wear a very similar head covering, closely resembling the shape of Euonymus europaeus fruits. While I never intended to get as closely involved with this genus, the very attractive nature of the fruits and seeds captivated me, and led me to produce this work. Prior to turning to the fruits of woody broadleaves, I primarily studied the winter buds and twigs of deciduous trees and shrubs (Schulz 1999, 2004, 2006b), a subject which was then little published, apart from the works by Schneider (1903) and Trelease (1931). -

GIANT ULLEUNG CELERY Stephen Barstow1, Malvik, March 2020
GIANT ULLEUNG CELERY 1 Stephen Barstow , Malvik, March 2020 Scientific name: Dystaenia takesimana Carrot family (Apiaceae) English: Seombadi, Sobadi, Dwaejipul, giant Ulleung celery, Korean pig-plant, wild celery, giant Korean celery Korean: 섬바디, 드와지풀 Norwegian: Ulleung kjempeselleri Swedish: Ullungloka, Vulkanloka The genus Dystaenia belongs to the carrot family or umbellifers (Apiaceae) and consists of two perennial species, one is a Japanese endemic (Dystaenia ibukiensis), and the other is endemic to a small island, Ulleung-do in Korea (Dystaenia takesimana). Genetic analysis (Pfosser et al., 2005) suggests that the larger D. takesimana evolved from D. ibukiensis rather than vice versa. The specific epithet takesimana is according to one reference to Takeshima Islet, which is disputed with the Japanese. Campanula takesimana is apparently found there. However, Takeshima island is also an alternative name for Ulleung-do, so this may be a misunderstanding. That Ulleung-do is Takeshima is confirmed on the following web site from the Oki Islands off Japan http://www.oki-geopark.jp/en/flowers-calendar/summer where it is stated that Dystaenia takesimana is also found there and is critically endangered: “This plant was designated as Cultural Property of Ama Town in 2012. It has only been discovered on the two isolated islands of Ama Town of the Oki Islands (Nakanoshima Island) and Ulleung-do Island of South Korea. It can be seen on the Akiya Coast in Nakanoshima Island. It is called Takeshima- shishiudo, as Ulleung-do was referred to as Takeshima” (see the map in Figure 1 for places mentioned here). Ulleung-do is a rocky steep-sided volcanic island some 120 km east of the coast of South Korea, the highest peak reaching 984m. -

The Genera of Bambusoideae (Gramineae) in the Southeastern United States Gordon C
Eastern Illinois University The Keep Faculty Research & Creative Activity Biological Sciences January 1988 The genera of Bambusoideae (Gramineae) in the southeastern United States Gordon C. Tucker Eastern Illinois University, [email protected] Follow this and additional works at: http://thekeep.eiu.edu/bio_fac Part of the Biology Commons Recommended Citation Tucker, Gordon C., "The eg nera of Bambusoideae (Gramineae) in the southeastern United States" (1988). Faculty Research & Creative Activity. 181. http://thekeep.eiu.edu/bio_fac/181 This Article is brought to you for free and open access by the Biological Sciences at The Keep. It has been accepted for inclusion in Faculty Research & Creative Activity by an authorized administrator of The Keep. For more information, please contact [email protected]. TUCKER, BAMBUSOIDEAE 239 THE GENERA OF BAMBUSOIDEAE (GRAMINEAE) IN THE SOUTHEASTERN UNITED STATESu GoRDON C. T ucKER3 Subfamily BAMBUSOIDEAE Ascherson & Graebner, Synop. Mitteleurop. Fl. 2: 769. 1902. Perennial or annual herbs or woody plants of tropical or temperate forests and wetlands. Rhizomes present or lacking. Stems erect or decumbent (some times rooting at the lower nodes); nodes glabrous, pubescent, or puberulent. Leaves several to many, glabrous to sparsely pubescent (microhairs bicellular); leaf sheaths about as long as the blades, open for over tf2 their length, glabrous; ligules wider than long, entire or fimbriate; blades petiolate or sessile, elliptic to linear, acute to acuminate, the primary veins parallel to-or forming an angle of 5-10• wi th-the midvein, transverse veinlets numerous, usually con spicuous, giving leaf surface a tessellate appearance; chlorenchyma not radiate (i.e., non-kranz; photosynthetic pathway C.,).