State of Delaware Invasive Plants Booklet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lonicera Maackii Amur Honeysuckle
Lonicera maackii (F.J. Ruprecht) C.J. Maximowicz Amur Honeysuckle (Caprifolium maackii, Xylosteon maackii) • Lonicera maackii is also known as Bush Honeysuckle; Amur Honeysuckle, which is a native of temperate Asia, forms a large deciduous irregular twiggy mound 12 to 15 tall with a similar or greater spread; growth rates can be rapid and plants can crowd out native vegetation; this is facilitated by the ability of Amur Honeysuckle to tolerate heavy shade until released; the gray pubescent buds of L. maackii can be distinguished from those of L. tatarica which are flattened and glabrous; the bluish green to medium green leaves of L. maackii are longer, 2 to 3 long, have mostly broadly cuneate to occasionally rounded bases, and acute to long acuminate tips while the medium to dark green leaves of L. tatarica are on average shorter, 1½ to 2½ long, with rounded to slightly cordate bases, and acute to short acuminate tips; new twigs have a whitish pubescence and may be flushed purple-red, later maturing to a gray-brown; excavated brown pith is present between the solid nodes; fall colors vary from almost nonexistent to a poor yellow; the tan-brown to gray- brown bark of old trunks develops vertical thin exfoliations. • A profusion of ¾ to 1 long white flowers are borne in spring, fading to a creamy yellow as they mature; flowers are generally reminiscent of those of L. japonica, flowers are produced in spring, occurring during or shortly after the foliage emerges; the flowers are followed by shiny, bright red, ¼to ½ diameter berries, produced mostly in one to two pairs per node, which hold into late winter if not eaten by birds or other wildlife. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Lonicera Spp
Species: Lonicera spp. http://www.fs.fed.us/database/feis/plants/shrub/lonspp/all.html SPECIES: Lonicera spp. Choose from the following categories of information. Introductory Distribution and occurrence Botanical and ecological characteristics Fire ecology Fire effects Fire case studies Management considerations References INTRODUCTORY SPECIES: Lonicera spp. AUTHORSHIP AND CITATION FEIS ABBREVIATION SYNONYMS NRCS PLANT CODE COMMON NAMES TAXONOMY LIFE FORM FEDERAL LEGAL STATUS OTHER STATUS AUTHORSHIP AND CITATION: Munger, Gregory T. 2005. Lonicera spp. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/ [2007, September 24]. FEIS ABBREVIATIONS: LONSPP LONFRA LONMAA LONMOR LONTAT LONXYL LONBEL SYNONYMS: None NRCS PLANT CODES [172]: LOFR LOMA6 LOMO2 LOTA LOXY 1 of 67 9/24/2007 4:44 PM Species: Lonicera spp. http://www.fs.fed.us/database/feis/plants/shrub/lonspp/all.html LOBE COMMON NAMES: winter honeysuckle Amur honeysuckle Morrow's honeysuckle Tatarian honeysuckle European fly honeysuckle Bell's honeysuckle TAXONOMY: The currently accepted genus name for honeysuckle is Lonicera L. (Caprifoliaceae) [18,36,54,59,82,83,93,133,161,189,190,191,197]. This report summarizes information on 5 species and 1 hybrid of Lonicera: Lonicera fragrantissima Lindl. & Paxt. [36,82,83,133,191] winter honeysuckle Lonicera maackii Maxim. [18,27,36,54,59,82,83,131,137,186] Amur honeysuckle Lonicera morrowii A. Gray [18,39,54,60,83,161,186,189,190,197] Morrow's honeysuckle Lonicera tatarica L. [18,38,39,54,59,60,82,83,92,93,157,161,186,190,191] Tatarian honeysuckle Lonicera xylosteum L. -

Invasive Plants of the Southeast Flyer
13 15 5 1 19 10 6 18 8 7 T o p 2 0 I n v a s i v e S p e c i e s 1. Chinese Privet, Ligustrum sinense 2. Nepalese Browntop, Microstegium vimineum 3. Autumn Olive, Elaeagnus umbellata 4. Chinese Wisteria, Wisteria sinensis & Japanese Wisteria, W. floribunda 5. Mimosa, Albizia julibrissin 6. Japanese Honeysuckle, Lonicera japonica 7. Amur Honeysuckle, Lonicera maackii 8. Multiflora Rose, Rosa multiflora 9. Hydrilla, Hydrilla verticillata 10. Kudzu, Pueraria montana 11. Golden Bamboo, Phyllostachys aurea 12. Oriental Bittersweet, Celastrus orbiculatus 13. English Ivy, Hedera helix 14. Tree-of-Heaven, Ailanthus altissima 15. Chinese Tallow, Sapium sebiferum 16. Chinese Princess Tree, Paulownia tomentosa 17. Japanese Knotweed, Polygonum cuspidatum 18. Silvergrass, Miscanthus sinensis 19. Thorny Olive, Elaeagnus pungens 20. Nandina, Nandina domestica The State Botanical Garden of Georgia and The Georgia Plant Conservation A l l i a n c e d e f i n i t i o n s you can help n a t i ve Avoid disturbing natural areas, including clearing of native vegetation. A native species is one that occurs in a particular region, ecosystem or habitat Know your plants. Find out if plants you without direct or indirect human action. grow have invasive tendencies. Do not use invasive species in landscaping, n o n - n a t i ve restoration, or for erosion control; use (alien, exotic, foreign, introduced, plants known not to be invasive in your area. non-indigenous) A species that occurs artificially in locations Control invasive plants on your land by beyond its known historical removing or managing them to prevent natural range. -

A Checklist of the Vascular Flora of the Mary K. Oxley Nature Center, Tulsa County, Oklahoma
Oklahoma Native Plant Record 29 Volume 13, December 2013 A CHECKLIST OF THE VASCULAR FLORA OF THE MARY K. OXLEY NATURE CENTER, TULSA COUNTY, OKLAHOMA Amy K. Buthod Oklahoma Biological Survey Oklahoma Natural Heritage Inventory Robert Bebb Herbarium University of Oklahoma Norman, OK 73019-0575 (405) 325-4034 Email: [email protected] Keywords: flora, exotics, inventory ABSTRACT This paper reports the results of an inventory of the vascular flora of the Mary K. Oxley Nature Center in Tulsa, Oklahoma. A total of 342 taxa from 75 families and 237 genera were collected from four main vegetation types. The families Asteraceae and Poaceae were the largest, with 49 and 42 taxa, respectively. Fifty-eight exotic taxa were found, representing 17% of the total flora. Twelve taxa tracked by the Oklahoma Natural Heritage Inventory were present. INTRODUCTION clayey sediment (USDA Soil Conservation Service 1977). Climate is Subtropical The objective of this study was to Humid, and summers are humid and warm inventory the vascular plants of the Mary K. with a mean July temperature of 27.5° C Oxley Nature Center (ONC) and to prepare (81.5° F). Winters are mild and short with a a list and voucher specimens for Oxley mean January temperature of 1.5° C personnel to use in education and outreach. (34.7° F) (Trewartha 1968). Mean annual Located within the 1,165.0 ha (2878 ac) precipitation is 106.5 cm (41.929 in), with Mohawk Park in northwestern Tulsa most occurring in the spring and fall County (ONC headquarters located at (Oklahoma Climatological Survey 2013). -

Duplication and Diversification of the LEAFY HULL STERILE1 and Oryza
Christensen and Malcomber EvoDevo 2012, 3:4 http://www.evodevojournal.com/content/3/1/4 RESEARCH Open Access Duplication and diversification of the LEAFY HULL STERILE1 and Oryza sativa MADS5 SEPALLATA lineages in graminoid Poales Ashley R Christensen1,2 and Simon T Malcomber1* Abstract Background: Gene duplication and the subsequent divergence in function of the resulting paralogs via subfunctionalization and/or neofunctionalization is hypothesized to have played a major role in the evolution of plant form. The LEAFY HULL STERILE1 (LHS1) SEPALLATA (SEP) genes have been linked with the origin and diversification of the grass spikelet, but it is uncertain 1) when the duplication event that produced the LHS1 clade and its paralogous lineage Oryza sativa MADS5 (OSM5) occurred, and 2) how changes in gene structure and/or expression might have contributed to subfunctionalization and/or neofunctionalization in the two lineages. Methods: Phylogenetic relationships among 84 SEP genes were estimated using Bayesian methods. RNA expression patterns were inferred using in situ hybridization. The patterns of protein sequence and RNA expression evolution were reconstructed using maximum parsimony (MP) and maximum likelihood (ML) methods, respectively. Results: Phylogenetic analyses mapped the LHS1/OSM5 duplication event to the base of the grass family. MP character reconstructions estimated a change from cytosine to thymine in the first codon position of the first amino acid after the Zea mays MADS3 (ZMM3) domain converted a glutamine to a stop codon in the OSM5 ancestor following the LHS1/OSM5 duplication event. RNA expression analyses of OSM5 co-orthologs in Avena sativa, Chasmanthium latifolium, Hordeum vulgare, Pennisetum glaucum, and Sorghum bicolor followed by ML reconstructions of these data and previously published analyses estimated a complex pattern of gain and loss of LHS1 and OSM5 expression in different floral organs and different flowers within the spikelet or inflorescence. -

Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Spring 5-2016 Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi Hanna M. Miller University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Biodiversity Commons, and the Botany Commons Recommended Citation Miller, Hanna M., "Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi" (2016). Honors Theses. 389. https://aquila.usm.edu/honors_theses/389 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi by Hanna Miller A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirement for the Degree of Bachelor of Science in the Department of Biological Sciences May 2016 ii Approved by _________________________________ Mac H. Alford, Ph.D., Thesis Adviser Professor of Biological Sciences _________________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences _________________________________ Ellen Weinauer, Ph.D., Dean Honors College iii Abstract The North American Coastal Plain contains some of the highest plant diversity in the temperate world. However, most of the region has remained unstudied, resulting in a lack of knowledge about the unique plant communities present there. -
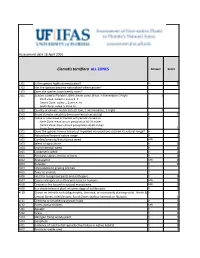
WRA.Datasheet.Template (Version 1) (Version 1).Xlsx
Assessment date 18 April 2016 Clematis terniflora ALL ZONES Answer Score 1.01 Is the species highly domesticated? n 0 1.02 Has the species become naturalised where grown? 1.03 Does the species have weedy races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality of climate match data (0-low; 1-intermediate; 2-high) 2 2.03 Broad climate suitability (environmental versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation y North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 1 2.05 Does the species have a history of repeated introductions outside its natural range? y 3.01 Naturalized beyond native range y 2 3.02 Garden/amenity/disturbance weed unk 3.03 Weed of agriculture n 0 3.04 Environmental weed y 4 3.05 Congeneric weed y 2 4.01 Produces spines, thorns or burrs n 0 4.02 Allelopathic unk 0 4.03 Parasitic n 0 4.04 Unpalatable to grazing animals ? 4.05 Toxic to animals ? 4.06 Host for recognised pests and pathogens n 0 4.07 Causes allergies or is otherwise toxic to humans unk 0 4.08 Creates a fire hazard in natural ecosystems unk 0 4.09 Is a shade tolerant plant at some stage of its life cycle n 0 4.10 Grows on infertile soils (oligotrophic, limerock, or excessively draining soils). -

Cereal Rust Bulletin Mailing List Next Season, Please Return the Enclosed Card by September 1, 1984
CEREAL RUST Report No: 1 BULLETIN April 17, 1984 From: Issued By: CEREAL RUST LABORATORY AGRICULTURAL RESEARCH SERVICE U, S. DEPARTMENT OF AGRICULTURE U. S. DEPARTMENT OF AGRICULTURE UNIVERSITY OF MINNESOTA, ST. PAUL 55108 (In cooperation with the Minnesota Agricultural Experiment Station) The cold winter and cool spring weather has slowed development of small grains to 1-2 weeks behind normal across the southern United States. In most of this region, moisture has been adequate but in south Texas the crop is under moisture stress. In the central and northern U.S. wheat growing areas, the winter was cold with spotty snow cover. In southern Nebraska and ~orthern Kansas, winter k i 11 i ng was more severe than normal due to the lack of snow cover. In the central plains, spring seeding of oats and barley is in full swing. In the northern plains, the initial planting of spring grains started but was delayed by rains. Warm drying weather should soon result in the resumption of planting. Wheat stem rust--In 1984, the first wheat stem rust was observed in McNair 701 disease detection plots at Victoria and Uvalde, Texas, experiment stations on April 7. At the Victoria site, the rust overwintered and with the increase in spring temperatures the rust increased to the point where it killed the plants. In the Uvalde plots, the rust infection was approximately three weeks old. Scattered pustules developed from spores that were deposited with rainfall. These spores probably originated some distance from the plot. These are the only reports of wheat stem rust in the United States at the present time. -

Native Nebraska Woody Plants
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS NATIVE NEBRASKA WOODY PLANTS Trees (Genus/Species – Common Name) 62. Atriplex canescens - four-wing saltbrush 1. Acer glabrum - Rocky Mountain maple 63. Atriplex nuttallii - moundscale 2. Acer negundo - boxelder maple 64. Ceanothus americanus - New Jersey tea 3. Acer saccharinum - silver maple 65. Ceanothus herbaceous - inland ceanothus 4. Aesculus glabra - Ohio buckeye 66. Cephalanthus occidentalis - buttonbush 5. Asimina triloba - pawpaw 67. Cercocarpus montanus - mountain mahogany 6. Betula occidentalis - water birch 68. Chrysothamnus nauseosus - rabbitbrush 7. Betula papyrifera - paper birch 69. Chrysothamnus parryi - parry rabbitbrush 8. Carya cordiformis - bitternut hickory 70. Cornus amomum - silky (pale) dogwood 9. Carya ovata - shagbark hickory 71. Cornus drummondii - roughleaf dogwood 10. Celtis occidentalis - hackberry 72. Cornus racemosa - gray dogwood 11. Cercis canadensis - eastern redbud 73. Cornus sericea - red-stem (redosier) dogwood 12. Crataegus mollis - downy hawthorn 74. Corylus americana - American hazelnut 13. Crataegus succulenta - succulent hawthorn 75. Euonymus atropurpureus - eastern wahoo 14. Fraxinus americana - white ash 76. Juniperus communis - common juniper 15. Fraxinus pennsylvanica - green ash 77. Juniperus horizontalis - creeping juniper 16. Gleditsia triacanthos - honeylocust 78. Mahonia repens - creeping mahonia 17. Gymnocladus dioicus - Kentucky coffeetree 79. Physocarpus opulifolius - ninebark 18. Juglans nigra - black walnut 80. Prunus besseyi - western sandcherry 19. Juniperus scopulorum - Rocky Mountain juniper 81. Rhamnus lanceolata - lanceleaf buckthorn 20. Juniperus virginiana - eastern redcedar 82. Rhus aromatica - fragrant sumac 21. Malus ioensis - wild crabapple 83. Rhus copallina - flameleaf (shining) sumac 22. Morus rubra - red mulberry 84. Rhus glabra - smooth sumac 23. Ostrya virginiana - hophornbeam (ironwood) 85. Rhus trilobata - skunkbush sumac 24. Pinus flexilis - limber pine 86. Ribes americanum - wild black currant 25. -

1 History of Vitaceae Inferred from Morphology-Based
HISTORY OF VITACEAE INFERRED FROM MORPHOLOGY-BASED PHYLOGENY AND THE FOSSIL RECORD OF SEEDS By IJU CHEN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2009 1 © 2009 Iju Chen 2 To my parents and my sisters, 2-, 3-, 4-ju 3 ACKNOWLEDGMENTS I thank Dr. Steven Manchester for providing the important fossil information, sharing the beautiful images of the fossils, and reviewing the dissertation. I thank Dr. Walter Judd for providing valuable discussion. I thank Dr. Hongshan Wang, Dr. Dario de Franceschi, Dr. Mary Dettmann, and Dr. Peta Hayes for access to the paleobotanical specimens in museum collections, Dr. Kent Perkins for arranging the herbarium loans, Dr. Suhua Shi for arranging the field trip in China, and Dr. Betsy R. Jackes for lending extant Australian vitaceous seeds and arranging the field trip in Australia. This research is partially supported by National Science Foundation Doctoral Dissertation Improvement Grants award number 0608342. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ...............................................................................................................4 LIST OF TABLES...........................................................................................................................9 LIST OF FIGURES .......................................................................................................................11 ABSTRACT...................................................................................................................................14 -

Caprifoliaceae, Dipsacales)
Syst. Biol. 59(3):322–341, 2010 c The Author(s) 2010. Published by Oxford University Press, on behalf of the Society of Systematic Biologists. All rights reserved. For Permissions, please email: [email protected] DOI:10.1093/sysbio/syq011 Advance Access publication on March 22, 2010 Combining Historical Biogeography with Niche Modeling in the Caprifolium Clade of Lonicera (Caprifoliaceae, Dipsacales) STEPHEN A.SMITH1,2,* AND MICHAEL J.DONOGHUE1 1Department of Ecology and Evolutionary Biology, Yale University, PO Box 208105, New Haven, CT 06520, USA; and 2National Evolutionary Synthesis Center, 2024 West Main Street, Suite A200, Durham, NC 27705, USA; *Correspondence to be sent to: National Evolutionary Synthesis Center, 2024 West Main Street, Suite A200, Durham, NC 27705, USA; E-mail:[email protected]. Received 15 September 2008; reviews returned 17 February 2009; accepted 9 December 2009 Associate Editor: Roberta J. Mason-Gamer Abstract.—The Lonicera clade Caprifolium contains 25 species distributed around the Northern Hemisphere, including in the Mediterranean climates of California and Europe.∼ We sequenced the second intron of LFY to help resolve relationships within the clade where the internal transcribed spacer and chloroplast markers had previously failed to do so. Divergence Downloaded from time estimation and biogeographic analyses over the posterior distribution of dated trees suggest that a widespread an- cestor was distributed across the Northern Hemisphere some 7–17 million years ago. Asian species form a sister group to a clade in which the European species are sister to the North American species. We use climatic niche modeling and divergence time estimates to explore the evolution of climate variables in the group.