The Orphan Receptor GPR17 Is Unresponsive to Uracil Nucleotides and Cysteinyl Leukotrienes S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Applying Screening Techniques to Two Orphan Gpcrs
Universidade de Lisboa Faculdade de Farmácia Deorphanization of receptors: Applying screening techniques to two orphan GPCRs Ana Catarina Rufas da Silva Santos Mestrado Integrado em Ciências Farmacêuticas 2019 Universidade de Lisboa Faculdade de Farmácia Deorphanization of receptors: Applying screening techniques to two orphan GPCRs Ana Catarina Rufas da Silva Santos Monografia de Mestrado Integrado em Ciências Farmacêuticas apresentada à Universidade de Lisboa através da Faculdade de Farmácia Orientadora: Ghazl Al Hamwi, PhD Student Co-Orientadora: Professora Doutora Elsa Maria Ribeiro dos Santos Anes, Professora Associada com Agregação em Microbiologia 2019 Abstract G-Protein Coupled Receptors represent one of the largest families of cellular receptors discovered and one of the main sources of attractive drug targets. In contrast, it also has a large number of understudied or orphan receptors. Pharmacological assays such as β-Arrestin recruitment assays, are one of the possible approaches for deorphanization of receptors. In this work, I applied the assay system previously mentioned to screen compounds in two orphan receptors, GRP37 and MRGPRX3. GPR37 has been primarily associated with a form of early onset Parkinsonism due to its’ expression patterns, and physiological role as substrate to ubiquitin E3, parkin. Although extensive literature regarding this receptor is available, the identification of a universally recognized ligand has not yet been possible. Two compounds were proposed as ligands, but both were met with controversy. These receptor association with Autosomal Recessive Juvenile Parkinson positions it as a very attractive drug target, and as such its’ deorphanization is a prime objective for investigators in this area. Regarding MRGPRX3 information is much scarcer. -

Nuclear Receptors — a Perspective from Drosophila
REVIEWS NUCLEAR RECEPTORS — A PERSPECTIVE FROM DROSOPHILA Kirst King-Jones and Carl S. Thummel Abstract | Nuclear receptors are ancient ligand-regulated transcription factors that control key metabolic and developmental pathways. The fruitfly Drosophila melanogaster has only 18 nuclear-receptor genes — far fewer than any other genetic model organism and representing all 6 subfamilies of vertebrate receptors. These unique attributes establish the fly as an ideal system for studying the regulation and function of nuclear receptors during development. Here, we review recent breakthroughs in our understanding of D. melanogaster nuclear receptors, and interpret these results in light of findings from their evolutionarily conserved vertebrate homologues. CLADE Classical signal-transduction pathways originate with a A wide range of small, lipophilic molecules function as A group of organisms that membrane-bound receptor. On binding its cognate nuclear-receptor ligands, including steroids, thyroid includes common ancestor and ligand, the receptor initiates a cascade of events in the hormone, retinoic acid and vitamin D. Not all nuclear all of its descendants, cytoplasm, eventually affecting specific transcription receptors, however, have a known natural ligand, and at representing a distinct branch on a phylogenetic tree. factors in the nucleus. In contrast to these often complex least some of these so-called orphan receptors can and convoluted pathways, members of the nuclear- function in a ligand-independent fashion3,4. receptor superfamily -

Genome-Wide Prediction of Small Molecule Binding to Remote
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.236729; this version posted August 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Genome-wide Prediction of Small Molecule Binding 2 to Remote Orphan Proteins Using Distilled Sequence 3 Alignment Embedding 1 2 3 4 4 Tian Cai , Hansaim Lim , Kyra Alyssa Abbu , Yue Qiu , 5,6 1,2,3,4,7,* 5 Ruth Nussinov , and Lei Xie 1 6 Ph.D. Program in Computer Science, The Graduate Center, The City University of New York, New York, 10016, USA 2 7 Ph.D. Program in Biochemistry, The Graduate Center, The City University of New York, New York, 10016, USA 3 8 Department of Computer Science, Hunter College, The City University of New York, New York, 10065, USA 4 9 Ph.D. Program in Biology, The Graduate Center, The City University of New York, New York, 10016, USA 5 10 Computational Structural Biology Section, Basic Science Program, Frederick National Laboratory for Cancer Research, 11 Frederick, MD 21702, USA 6 12 Department of Human Molecular Genetics and Biochemistry, Sackler School of Medicine, Tel Aviv University, Tel 13 Aviv, Israel 7 14 Helen and Robert Appel Alzheimer’s Disease Research Institute, Feil Family Brain & Mind Research Institute, Weill 15 Cornell Medicine, Cornell University, New York, 10021, USA * 16 [email protected] 17 July 27, 2020 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.236729; this version posted August 5, 2020. -

Edinburgh Research Explorer
Edinburgh Research Explorer International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list Citation for published version: Davenport, AP, Alexander, SPH, Sharman, JL, Pawson, AJ, Benson, HE, Monaghan, AE, Liew, WC, Mpamhanga, CP, Bonner, TI, Neubig, RR, Pin, JP, Spedding, M & Harmar, AJ 2013, 'International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands', Pharmacological reviews, vol. 65, no. 3, pp. 967-86. https://doi.org/10.1124/pr.112.007179 Digital Object Identifier (DOI): 10.1124/pr.112.007179 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Pharmacological reviews Publisher Rights Statement: U.S. Government work not protected by U.S. copyright General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 1521-0081/65/3/967–986$25.00 http://dx.doi.org/10.1124/pr.112.007179 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:967–986, July 2013 U.S. -
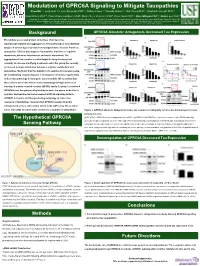
Background the Hypothetical GPRC6A Sensing Pathway
Modulation of GPRC6A Signaling to Mitigate Tauopathies Chao Ma1,2, Jerry Hunt1,3, Leslie Sandusky PhD1,3, William Fraser1,3, Ronald Alvarez1,3, Dali Zheng PhD1,4, Siddharth Kamath PhD1,2, Chad Dickey PhD1,4, Hans Bräuner-Osborne PhD5, Daniel Sejer Pedersen PhD5, Kevin Nash PhD1,2, Dave Morgan PhD1,2, Daniel Lee PhD1,3 1:Byrd Alzheimer‘s Institute, University of South Florida, Tampa, FL 33613, USA. 2:Morsani College of Medicine, Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, FL 33612, USA. 3:College of Pharmacy, Department of Pharmaceutical Sciences, University of South Florida, Tampa, FL 33612, USA. 4:Morsani College of Medicine, Department of Molecular Medicine, University of South Florida, Tampa, FL 33612, USA. 5:Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Jagtvej 162, 2100 Copenhagen, Denmark Background GPRC6A Allosteric Antagonists Decreased Tau Expression Microtubule associated protein named tau, often becomes hyperphosphorylated and aggregates to form pathological neurofibrillary tangles in several age-associated neurodegenerative diseases known as tauopathies. Clinical phenotypes of tauopathies manifest as cognitive impairment, behavior disturbances and motor impairment. The aggregation of tau remains a central target for drug discovery, but currently no disease-modifying treatments exist. Our group has recently uncovered a unique interaction between L-arginine metabolism and tauopathies. We found that the depletion of L-arginine by overexpressing the metabolizing enzyme arginase 1 and arginine deiminase significantly reduced tau pathology in transgenic mouse models. We speculate that these effects associate with increased autophagy through amino acid sensing. G protein-coupled receptor (GPCR), family C, group 6, member A (GPRC6A) was deorphanized by binding to basic L-α amino acids, like L- arginine. -

G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte
www.nature.com/scientificreports OPEN G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Diferentiation in Response Received: 11 May 2017 Accepted: 2 October 2017 to Lysolecithin-Induced Published: xx xx xxxx Demyelination Changqing Lu1,2, Lihua Dong2, Hui Zhou3, Qianmei Li3, Guojiao Huang3, Shu jun Bai3 & Linchuan Liao1 Oligodendrocytes are the myelin-producing cells of the central nervous system (CNS). A variety of brain disorders from “classical” demyelinating diseases, such as multiple sclerosis, stroke, schizophrenia, depression, Down syndrome and autism, are shown myelination defects. Oligodendrocyte myelination is regulated by a complex interplay of intrinsic, epigenetic and extrinsic factors. Gpr17 (G protein- coupled receptor 17) is a G protein-coupled receptor, and has been identifed to be a regulator for oligodendrocyte development. Here, we demonstrate that the absence of Gpr17 enhances remyelination in vivo with a toxin-induced model whereby focal demyelinated lesions are generated in spinal cord white matter of adult mice by localized injection of LPC(L-a-lysophosphatidylcholine). The increased expression of the activated form of Erk1/2 (phospho-Erk1/2) in lesion areas suggested the potential role of Erk1/2 activity on the Gpr17-dependent modulation of myelination. The absence of Gpr17 enhances remyelination is correlate with the activated Erk1/2 (phospho-Erk1/2).Being a membrane receptor, Gpr17 represents an ideal druggable target to be exploited for innovative regenerative approaches to acute and chronic CNS diseases. Oligodendrocytes are the myelin-producing cells of the central nervous system (CNS), and as such, wrap layers of lipid-dense insulating myelin around axons1. Mature oligodendrocytes have also been shown to provide met- abolic support to axons through transport systems within myelin, which may help prevent neurodegeneration2. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

A Key Interfacial Residue Identified with In-Cell Structure Characterization Of
bioRxiv preprint doi: https://doi.org/10.1101/664094; this version posted June 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 A key interfacial residue identified with in-cell structure 2 characterization of a class A GPCR dimer 3 4 Ju Yang2,7,8, Zhou Gong2,8, Yun-Bi Lu1,8, Chan-Juan Xu3, Tao-Feng Wei1, Men-Shi Yang2, Tian-Wei 5 Zhan4, Yu-Hong Yang2, Li Lin3, Jian-Feng Liu3*, Chun Tang2,5*, Wei-Ping Zhang1,6* 6 7 1Department of Pharmacology, Key Laboratory of Medical Neurobiology of Ministry of Health of China, 8 Zhejiang University School of Medicine, Hangzhou, Zhejiang 310058, China. 9 2Key Laboratory of Magnetic Resonance in Biological Systems of the Chinese Academy of Sciences, 10 State Key Laboratory of Magnetic Resonance and Atomic Molecular Physics, National Center for 11 Magnetic Resonance at Wuhan, Wuhan Institute of Physics and Mathematics of the Chinese Academy of 12 Sciences, Wuhan, Hubei 430071, China.College of Life Science and Technology, Collaborative 13 Innovation Center for Genetics and Development, Key Laboratory of Molecular Biophysics of Ministry 14 of Education, Huazhong University of Science and Technology, Wuhan, Hubei 430074, China. 15 4Department of Thoracic Surgery, the Second Affiliated Hospital of Zhejiang University School of 16 Medicine, Zhejiang 310009, China. 17 5Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, 18 Wuhan, Hubei Province 430074, China. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

Purinergic Receptors Brian F
Chapter 21 Purinergic receptors Brian F. King and Geoffrey Burnstock 21.1 Introduction The term purinergic receptor (or purinoceptor) was first introduced to describe classes of membrane receptors that, when activated by either neurally released ATP (P2 purinoceptor) or its breakdown product adenosine (P1 purinoceptor), mediated relaxation of gut smooth muscle (Burnstock 1972, 1978). P2 purinoceptors were further divided into five broad phenotypes (P2X, P2Y, P2Z, P2U, and P2T) according to pharmacological profile and tissue distribution (Burnstock and Kennedy 1985; Gordon 1986; O’Connor et al. 1991; Dubyak 1991). Thereafter, they were reorganized into families of metabotropic ATP receptors (P2Y, P2U, and P2T) and ionotropic ATP receptors (P2X and P2Z) (Dubyak and El-Moatassim 1993), later redefined as extended P2Y and P2X families (Abbracchio and Burnstock 1994). In the early 1990s, cDNAs were isolated for three heptahelical proteins—called P2Y1, P2Y2, and P2Y3—with structural similarities to the rhodopsin GPCR template. At first, these three GPCRs were believed to correspond to the P2Y, P2U, and P2T receptors. However, the com- plexity of the P2Y receptor family was underestimated. At least 15, possibly 16, heptahelical proteins have been associated with the P2Y receptor family (King et al. 2001, see Table 21.1). Multiple expression of P2Y receptors is considered the norm in all tissues (Ralevic and Burnstock 1998) and mixtures of P2 purinoceptors have been reported in central neurones (Chessell et al. 1997) and glia (King et al. 1996). The situation is compounded by P2Y protein dimerization to generate receptor assemblies with subtly distinct pharmacological proper- ties from their constituent components (Filippov et al. -

G Protein-Coupled Receptors Function As Cell Membrane Receptors for the Steroid Hormone 20-Hydroxyecdysone Xiao-Fan Zhao
Zhao Cell Communication and Signaling (2020) 18:146 https://doi.org/10.1186/s12964-020-00620-y REVIEW Open Access G protein-coupled receptors function as cell membrane receptors for the steroid hormone 20-hydroxyecdysone Xiao-Fan Zhao Abstract G protein-coupled receptors (GPCRs) are cell membrane receptors for various ligands. Recent studies have suggested that GPCRs transmit animal steroid hormone signals. Certain GPCRs have been shown to bind steroid hormones, for example, G protein-coupled estrogen receptor 1 (GPER1) binds estrogen in humans, and Drosophila dopamine/ecdysteroid receptor (DopEcR) binds the molting hormone 20-hydroxyecdysone (20E) in insects. This review summarizes the research progress on GPCRs as animal steroid hormone cell membrane receptors, including the nuclear and cell membrane receptors of steroid hormones in mammals and insects, the 20E signaling cascade via GPCRs, termination of 20E signaling, and the relationship between genomic action and the nongenomic action of 20E. Studies indicate that 20E induces a signal via GPCRs to regulate rapid cellular responses, including rapid Ca2+ release from the endoplasmic reticulum and influx from the extracellular medium, as well as rapid protein phosphorylation and subcellular translocation. 20E via the GPCR/Ca2+/PKC/signaling axis and the GPCR/cAMP/PKA- signaling axis regulates gene transcription by adjusting transcription complex formation and DNA binding activity. GPCRs can bind 20E in the cell membrane and after being isolated, suggesting GPCRs as cell membrane receptors of 20E. This review deepens our understanding of GPCRs as steroid hormone cell membrane receptors and the GPCR-mediated signaling pathway of 20E (20E-GPCR pathway), which will promote further study of steroid hormone signaling via GPCRs, and presents GPCRs as targets to explore new pharmaceutical materials to treat steroid hormone-related diseases or control pest insects. -

GPR17 Is a Negative Regulator of the Cysteinyl Leukotriene 1 Receptor Response to Leukotriene D4 Akiko Maekawaa,B, Barbara Balestrieria,B, K
GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4 Akiko Maekawaa,b, Barbara Balestrieria,b, K. Frank Austena,b,1, and Yoshihide Kanaokaa,b,1 aDepartment of Medicine, Harvard Medical School, Boston, MA 02115; and bDivision of Rheumatology, Immunology, and Allergy, Brigham and Women’s Hospital, One Jimmy Fund Way, Boston, MA 02115 Contributed by K. Frank Austen, May 20, 2009 (sent for review May 5, 2009) The cysteinyl leukotrienes (cys-LTs) are proinflammatory lipid me- CysLT2RtobeLTD4 Ͼ LTC4 Ͼ LTE4 and LTC4 ϭ LTD4 Ͼ diators acting on the type 1 cys-LT receptor (CysLT1R) to mediate LTE4, respectively. The findings that these receptors are ex- smooth muscle constriction and vascular permeability. GPR17, a G pressed not only on human smooth muscle but also on bone protein-coupled orphan receptor with homology to the P2Y and marrow-derived cells of the innate and adaptive immune systems cys-LT receptors, failed to mediate calcium flux in response to revealed a potential for involvement of the cys-LT/CysLTR leukotriene (LT) D4 with stable transfectants. However, in stable pathway in the infiltrating cells of the inflammatory response cotransfections of 6؋His-tagged GPR17 with Myc-tagged CysLT1R, (18, 19). We and others subsequently reported that the mouse the robust CysLT1R-mediated calcium response to LTD4 was abol- CysLT1R can function as a receptor for LTD4 in transfected cells ished. The membrane expression of the CysLT1R analyzed by FACS with a ligand preference similar to that of the human CysLT1R with anti-Myc Ab was not reduced by the cotransfection, yet both (20, 21) and that the mouse CysLT2R exhibits a ligand profile of LTD4-elicited ERK phosphorylation and the specific binding of LTC4 Ն LTD4 Ͼ LTE4 (21, 22).