In Vivo Cell Biological Screening Identifies an Endocytic Capture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1933.Full.Pdf
0270.6474/84/0408-1933$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 8, pp. 1933-1943 Printed in U.S.A. August 1984 PARTIAL PURIFICATION AND FUNCTIONAL IDENTIFICATION OF A CALMODULIN-ACTIVATED, ADENOSINE 5’-TRIPHOSPHATE-DEPENDENT CALCIUM PUMP FROM SYNAPTIC PLASMA MEMBRANES1 DIANE M. PAPAZIAN,* HANNAH RAHAMIMOFF,$ AND STANLEY M. GOLDIN§’ Departments of *Biological Chemistry and $Pharmacology, Harvard Medical School, Boston, Massachusetts 02115 and *Department of Biochemistry, Hadassah Medical School, Hebrew University, Jerusalem, Israel Received July 18, 1983; Revised February 27, 1984; Accepted February 28, 1984 Abstract Synaptic plasma membranes isolated from rat brain contain a calmodulin-activated Ca2+ pump. It has been purified 80- to 160-fold by solubilization with Triton X-100 and affinity chromatography on a calmodulin- Sepharose 4B column. After reconstitution into phospholipid vesicles, the affinity-purified pump efficiently catalyzed ATP dependent Ca2+ transport, which was activated 7- to g-fold by calmodulin. The major protein component of the affinity-purified preparation had a M, = 140,000; it was virtually the only band visualized on a Coomassie blue-stained SDS polyacrylamide gel. It has been identified as the Ca”’ pump by two functional criteria. First, it was phosphorylated by [y-““P]ATP in a Ca’+-dependent manner; the phosphorylated protein had the chemical reactivity of an acyl phosphate, characteristic of the phosphorylated intermediates of ion-transporting ATPases. Second, the protein was enriched by transport-specific fractiona- tion, a density gradient procedure which uses the transport properties of the reconstituted Ca2+ pump as a physical tool for its purification. -

Hedgehog Signaling Modulates Cholesterol Homeostasis in Chondrocytes and in Osteoarthritis
HEDGEHOG SIGNALING MODULATES CHOLESTEROL HOMEOSTASIS IN CHONDROCYTES AND IN OSTEOARTHRITIS by Shabana Amanda Ali A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Institute of Medical Science University of Toronto © Copyright by Shabana Amanda Ali 2014 HEDGEHOG SIGNALING MODULATES CHOLESTEROL HOMEOSTASIS IN CHONDROCYTES AND IN OSTEOARTHRITIS Shabana Amanda Ali Doctor of Philosophy Institute of Medical Science University of Toronto 2014 Abstract Osteoarthritis (OA) is a common degenerative disease of the joint that is characterized by degradation and calcification of articular cartilage, and subchondral bone changes. Hedgehog (Hh) signaling is known to be activated in human and murine OA. Since Hh signaling regulates Gli‐mediated gene expression, we identified Hh target genes that are expressed in chondrocytes. Microarray analyses were performed to detect changes in gene expression when the Hh pathway was modulated in human OA cartilage samples. Results from the Affymetrix Human Gene 1.0 ST microarray were analyzed for differentially expressed genes from three patient samples. Using Ingenuity® Pathway analysis, several genes known to be involved in sterol homeostasis were found to be modulated with Hh inhibition. We hypothesized that Hh signaling regulates cholesterol biosynthesis in chondrocytes, and that modulating cholesterol homeostasis impacts the severity of OA. To investigate the function of cholesterol in the cartilage, mice with chondrocyte‐specific cholesterol accumulation were generated. This was achieved by excising Insig1 and Insig2, major negative regulators of cholesterol homeostasis. Over time, mice with chondrocyte‐specific cholesterol accumulation exhibited impaired growth of the long bones. With aging or surgically induced joint instability, these mice ii developed more severe OA than control littermates. -

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

When STING Meets Viruses: Sensing, Trafficking Andesponse R
Washington University School of Medicine Digital Commons@Becker Open Access Publications 1-1-2020 When STING meets viruses: Sensing, trafficking andesponse r Zhaohe Li Siqi Cai Yutong Sun Li Li Siyuan Ding See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Authors Zhaohe Li, Siqi Cai, Yutong Sun, Li Li, Siyuan Ding, and Xin Wang fimmu-11-02064 September 25, 2020 Time: 19:58 # 1 REVIEW published: 29 September 2020 doi: 10.3389/fimmu.2020.02064 When STING Meets Viruses: Sensing, Trafficking and Response Zhaohe Li1, Siqi Cai1, Yutong Sun1, Li Li1,2,3, Siyuan Ding4 and Xin Wang1,2,3* 1 Key Laboratory of Marine Drugs of Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China, 2 Center for Innovation Marine Drug Screening and Evaluation, Pilot National Laboratory for Marine Science and Technology, Qingdao, China, 3 Marine Biomedical Research Institute of Qingdao, Qingdao, China, 4 Department of Molecular Microbiology, School of Medicine, Washington University in St. Louis, St. Louis, MO, United States To effectively defend against microbial pathogens, the host cells mount antiviral innate immune responses by producing interferons (IFNs), and hundreds of IFN-stimulated genes (ISGs). Upon recognition of cytoplasmic viral or bacterial DNAs and abnormal endogenous DNAs, the DNA sensor cGAS synthesizes 2’,3’-cGAMP that induces STING (stimulator of interferon genes) undergoing conformational changes, cellular trafficking, and the activation of downstream factors. Therefore, STING plays a pivotal role in preventing microbial pathogen infection by sensing DNAs during pathogen invasion. This review is dedicated to the recent advances in the dynamic regulations of STING activation, intracellular trafficking, and post-translational modifications (PTMs) Edited by: by the host and microbial proteins. -

Sorting Nexins in Protein Homeostasis Sara E. Hanley1,And Katrina F
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 6 November 2020 doi:10.20944/preprints202011.0241.v1 Sorting nexins in protein homeostasis Sara E. Hanley1,and Katrina F. Cooper2* 1Department of Molecular Biology, Graduate School of Biomedical Sciences, Rowan University, Stratford, NJ, 08084, USA 1 [email protected] 2 [email protected] * [email protected] Tel: +1 (856)-566-2887 1Department of Molecular Biology, Graduate School of Biomedical Sciences, Rowan University, Stratford, NJ, 08084, USA Abstract: Sorting nexins (SNXs) are a highly conserved membrane-associated protein family that plays a role in regulating protein homeostasis. This family of proteins is unified by their characteristic phox (PX) phosphoinositides binding domain. Along with binding to membranes, this family of SNXs also comprises a diverse array of protein-protein interaction motifs that are required for cellular sorting and protein trafficking. SNXs play a role in maintaining the integrity of the proteome which is essential for regulating multiple fundamental processes such as cell cycle progression, transcription, metabolism, and stress response. To tightly regulate these processes proteins must be expressed and degraded in the correct location and at the correct time. The cell employs several proteolysis mechanisms to ensure that proteins are selectively degraded at the appropriate spatiotemporal conditions. SNXs play a role in ubiquitin-mediated protein homeostasis at multiple levels including cargo localization, recycling, degradation, and function. In this review, we will discuss the role of SNXs in three different protein homeostasis systems: endocytosis lysosomal, the ubiquitin-proteasomal, and the autophagy-lysosomal system. The highly conserved nature of this protein family by beginning with the early research on SNXs and protein trafficking in yeast and lead into their important roles in mammalian systems. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Spatiotemporal Profiling of Cytosolic Signaling Complexes in Living Cells by Selective Proximity Proteomics
ARTICLE https://doi.org/10.1038/s41467-020-20367-x OPEN Spatiotemporal profiling of cytosolic signaling complexes in living cells by selective proximity proteomics Mi Ke1,6, Xiao Yuan1,6,AnHe1, Peiyuan Yu 1, Wendong Chen1, Yu Shi2, Tony Hunter 2, Peng Zou 3 & ✉ Ruijun Tian 1,4,5 1234567890():,; Signaling complexes are often organized in a spatiotemporal manner and on a minute timescale. Proximity labeling based on engineered ascorbate peroxidase APEX2 pioneered in situ capture of spatiotemporal membrane protein complexes in living cells, but its appli- cation to cytosolic proteins remains limited due to the high labeling background. Here, we develop proximity labeling probes with increased labeling selectivity. These probes, in combination with label-free quantitative proteomics, allow exploring cytosolic protein assemblies such as phosphotyrosine-mediated protein complexes formed in response to minute-scale EGF stimulation. As proof-of-concept, we systematically profile the spatio- temporal interactome of the EGFR signaling component STS1. For STS1 core complexes, our proximity proteomics approach shows comparable performance to affinity purification-mass spectrometry-based temporal interactome profiling, while also capturing additional— especially endosomally-located—protein complexes. In summary, we provide a generic approach for exploring the interactome of mobile cytosolic proteins in living cells at a tem- poral resolution of minutes. 1 Department of Chemistry, School of Science, Southern University of Science and Technology, Shenzhen, China. 2 Molecular and Cell Biology Laboratory, Salk Institute for Biological Studies, La Jolla, CA, USA. 3 College of Chemistry and Molecular Engineering, Peking University, Beijing, China. 4 Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, Southern University of Science and Technology, Shenzhen, China. -

Sorting Nexin-4 Regulates Β-Amyloid Production by Modulating Β-Site
Kim et al. Alzheimer's Research & Therapy (2017) 9:4 DOI 10.1186/s13195-016-0232-8 RESEARCH Open Access Sorting nexin-4 regulates β-amyloid production by modulating β-site-activating cleavage enzyme-1 Na-Young Kim1,2,3,4†, Mi-Hyang Cho1,2,3,4†, Se-Hoon Won1,2,3,4, Hoe-Jin Kang1,2,3,4, Seung-Yong Yoon1,2,3,4* and Dong-Hou Kim1,2,3,4* Abstract Background: Amyloid precursor protein (APP) is cleaved by β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) to produce β-amyloid (Aβ), a critical pathogenic peptide in Alzheimer’s disease (AD). Aβ generation can be affected by the intracellular trafficking of APP or its related secretases, which is thus important to understanding its pathological alterations. Although sorting nexin (SNX) family proteins regulate this trafficking, the relevance and role of sorting nexin-4 (SNX4) regarding AD has not been studied yet. Methods: In this study, human brain tissue and APP/PS1 mouse brain tissue were used to check the disease relevance of SNX4. To investigate the role of SNX4 in AD pathogenesis, several experiments were done, such as coimmunoprecipitation, Western blotting, immunohistochemistry, and gradient fractionation. Results: We found that SNX4 protein levels changed in the brains of patients with AD and of AD model mice. Overexpression of SNX4 significantly increased the levels of BACE1 and Aβ. Downregulation of SNX4 had the opposite effect. SNX4 interacts with BACE1 and prevents BACE1 trafficking to the lysosomal degradation system, resulting in an increased half-life of BACE1 and increased production of Aβ. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Mir-17-92 Fine-Tunes MYC Expression and Function to Ensure
ARTICLE Received 31 Mar 2015 | Accepted 22 Sep 2015 | Published 10 Nov 2015 DOI: 10.1038/ncomms9725 OPEN miR-17-92 fine-tunes MYC expression and function to ensure optimal B cell lymphoma growth Marija Mihailovich1, Michael Bremang1, Valeria Spadotto1, Daniele Musiani1, Elena Vitale1, Gabriele Varano2,w, Federico Zambelli3, Francesco M. Mancuso1,w, David A. Cairns1,w, Giulio Pavesi3, Stefano Casola2 & Tiziana Bonaldi1 The synergism between c-MYC and miR-17-19b, a truncated version of the miR-17-92 cluster, is well-documented during tumor initiation. However, little is known about miR-17-19b function in established cancers. Here we investigate the role of miR-17-19b in c-MYC-driven lymphomas by integrating SILAC-based quantitative proteomics, transcriptomics and 30 untranslated region (UTR) analysis upon miR-17-19b overexpression. We identify over one hundred miR-17-19b targets, of which 40% are co-regulated by c-MYC. Downregulation of a new miR-17/20 target, checkpoint kinase 2 (Chek2), increases the recruitment of HuR to c- MYC transcripts, resulting in the inhibition of c-MYC translation and thus interfering with in vivo tumor growth. Hence, in established lymphomas, miR-17-19b fine-tunes c-MYC activity through a tight control of its function and expression, ultimately ensuring cancer cell homeostasis. Our data highlight the plasticity of miRNA function, reflecting changes in the mRNA landscape and 30 UTR shortening at different stages of tumorigenesis. 1 Department of Experimental Oncology, European Institute of Oncology, Via Adamello 16, Milan 20139, Italy. 2 Units of Genetics of B cells and lymphomas, IFOM, FIRC Institute of Molecular Oncology Foundation, Milan 20139, Italy. -
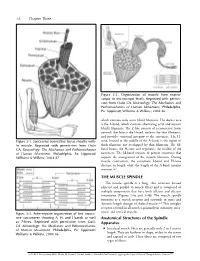
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles.