Zhi CHEN 1, Xiaoyan WANG 2, Jie ZHANG 3, Yuan LI 4, Tianxiang GAO 2, 5*, and Longshan LIN 4*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the Year 2016
www.shark-references.com Version 13.01.2017 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2016 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 13.01.2017 Abstract: This paper contains a collection of 803 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2016. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2016, list of descriptions of extinct and extant species from 2016, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide information on the geographic and depth distribution of newly described species, i.e. the type specimens from the year 1990- 2016 in a hot spot analysis. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, -

Checklist of Philippine Chondrichthyes
CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 Checklist of Philippine chondrichthyes. Bibliography. ISBN 1 876996 95 1. 1. Chondrichthyes - Philippines. 2. Sharks - Philippines. 3. Stingrays - Philippines. I. Compagno, Leonard Joseph Victor. II. CSIRO. Marine Laboratories. (Series : Report (CSIRO. Marine Laboratories) ; 243). 597.309599 1 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V.1, Last, P.R.2, Stevens, J.D.2, and Alava, M.N.R.3 1 Shark Research Center, South African Museum, Iziko–Museums of Cape Town, PO Box 61, Cape Town, 8000, South Africa 2 CSIRO Marine Research, GPO Box 1538, Hobart, Tasmania, 7001, Australia 3 Species Conservation Program, WWF-Phils., Teachers Village, Central Diliman, Quezon City 1101, Philippines (former address) ABSTRACT Since the first publication on Philippines fishes in 1706, naturalists and ichthyologists have attempted to define and describe the diversity of this rich and biogeographically important fauna. The emphasis has been on fishes generally but these studies have also contributed greatly to our knowledge of chondrichthyans in the region, as well as across the broader Indo–West Pacific. An annotated checklist of cartilaginous fishes of the Philippines is compiled based on historical information and new data. A Taiwanese deepwater trawl survey off Luzon in 1995 produced specimens of 15 species including 12 new records for the Philippines and a few species new to science. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

2020/016 Dated 28 February 2020 in the Language and Format in Which They Were Received
AC31 Doc. 25 Annex 2 Compilation of responses from Parties to Notification to the Parties No. 2020/016 dated 28 February 2020 in the language and format in which they were received Contents Cambodia ………………………………………………………………………………………………………………………………………....…1 Canada ………………………………………………………………………………………………………………………………..………..……..3 Colombia …………………………………………………………………………………………………………………………………….………..5 Costa Rica …………………………………………………………………………………………………………………………..………..………15 Croatia ……………………………………………………………………………………………………………………………..………..……….35 European Union ………………………………………………………………………………………………………..……………..………….36 Indonesia ………………………………………………………………………………………………………………………………………..…..43 Israel ……………………………………………………………………………………………………………………………………………..…….51 Italy ……………………………………………………………………………………………………………………………………………………..55 Japan ……………………………………………………………………………………………………………………………………………….….60 Mexico ………………………………………………………………………………………………………………………………………………..66 Monaco ……………………………………………………………………………………………………………………………………………….69 Netherlands …………………………………………………………………………………………………………………………………………73 New Zealand ………………………………………………………………………………………………………………………………………..77 Oceania Parties …………………………………………………………………………………………………………………………………….79 Peru ……………………………………………………………………………………………………………………………………………………..83 Senegal ………………………………………………………………………………………………………………………………………………..89 Thailand ……………………………………………………………………………………………………………………………………………….91 United States of America ……………………………………………………………………………………………………………………146 Cambodia AC31 Doc. 25 Annex 2 Canada Canadian Response to CITES Notification 2020/016 -

Database of Bibliography of Living/Fossil
www.shark-references.com Version 16.01.2018 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2017 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 DOI: 10.13140/RG.2.2.32409.72801 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 16.01.2018 Abstract: This paper contains a collection of 817 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2017. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2017, list of descriptions of extinct and extant species from 2017, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide data information on the geographic and depth distribution of newly described species, i.e. the type specimens from the years 1990 to 2017 in a hot spot analysis. New in this year's POTY is the subheader "biodiversity" comprising a complete list of all valid chimaeriform, selachian and batoid species, as well as a list of the top 20 most researched chondrichthyan species. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, possible errors cannot entirely be excluded. -

Guide to the Coastal Marine Fishes of California
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 157 GUIDE TO THE COASTAL MARINE FISHES OF CALIFORNIA by DANIEL J. MILLER and ROBERT N. LEA Marine Resources Region 1972 ABSTRACT This is a comprehensive identification guide encompassing all shallow marine fishes within California waters. Geographic range limits, maximum size, depth range, a brief color description, and some meristic counts including, if available: fin ray counts, lateral line pores, lateral line scales, gill rakers, and vertebrae are given. Body proportions and shapes are used in the keys and a state- ment concerning the rarity or commonness in California is given for each species. In all, 554 species are described. Three of these have not been re- corded or confirmed as occurring in California waters but are included since they are apt to appear. The remainder have been recorded as occurring in an area between the Mexican and Oregon borders and offshore to at least 50 miles. Five of California species as yet have not been named or described, and ichthyologists studying these new forms have given information on identification to enable inclusion here. A dichotomous key to 144 families includes an outline figure of a repre- sentative for all but two families. Keys are presented for all larger families, and diagnostic features are pointed out on most of the figures. Illustrations are presented for all but eight species. Of the 554 species, 439 are found primarily in depths less than 400 ft., 48 are meso- or bathypelagic species, and 67 are deepwater bottom dwelling forms rarely taken in less than 400 ft. -
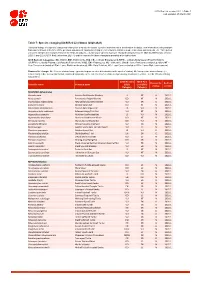
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Family Platyrhinidae P.R
13 FANRAYS Family Platyrhinidae P.R. Last & B. Séret Fanrays are small to medium-sized rays (adults from 30–90 cm TL) with a firm body, large, flattened subcircular to shovel-shaped disc and an elongate tapering tail. Pectoral-fin rays, which form the disc, extend forward to snout tip and beyond pelvic-fin origins. Nostrils are positioned close to the mouth, and eyes are narrowly separated and well removed from disc margin. Anterior nasal flaps short and not joined together to form a broad, flap-like nasal curtain. No dermal folds on spiracles. Upper surface with sharp thorns or enlarged denticles on head, shoulders and in row along mid-line of body. Two similar dorsal fins located close together on tail and positioned well behind pelvic fins. Caudal fin elongate and without a distinct lower lobe. Tail slender, abruptly narrower than trunk, lateral folds well developed, and lacking a stinging spine. Colour plain or with transverse stripes, but lacking pectoral ocelli. Ventral surface usually uniformly white. This small ray family includes 2 genera and 4 valid species. Panrays (Zanobatidae) and some banjo rays (Trygonorrhinidae) have a similar body shape but their dorsal fins are positioned relatively further forward on the tail. Fanrays occur in cool-temperate to tropical continental parts of the North-West and Eastern Central Pacific. Demersal in shallow water, living on a variety of soft substrates from nearshore to at least a depth of 137 m. None of the species ventures into freshwater. All species are ovoviviparous. Life histories mostly little known, they feed primarily on small marine invertebrates, including crustaceans, molluscs and worms. -

Myliobatiformes: Gymnuridae), with the Description of Two New Species
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2017 Age, Growth and Reproduction of Western North Atlantic Butterfly Rays (Myliobatiformes: Gymnuridae), with the Description of Two New Species Kristene Teal Parsons College of William and Mary - Virginia Institute of Marine Science, [email protected] Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Aquaculture and Fisheries Commons, Biology Commons, Marine Biology Commons, and the Zoology Commons Recommended Citation Parsons, Kristene Teal, "Age, Growth and Reproduction of Western North Atlantic Butterfly Rays (Myliobatiformes: Gymnuridae), with the Description of Two New Species" (2017). Dissertations, Theses, and Masters Projects. Paper 1516639565. http://dx.doi.org/doi:10.21220/V58T7S This Dissertation is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Age, Growth and Reproduction of Western North Atlantic Butterfly Rays (Myliobatiformes: Gymnuridae), with the Description of Two New Species A Dissertation Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Kristene Teal Parsons August 2017 APPROVAL PAGE This dissertation is submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Kristene Teal Parsons Approved by the Committee, May 2017 Robert J. Latour, Ph.D. Committee Chair / Advisor Eric J. -

HANDBOOK of FISH BIOLOGY and FISHERIES Volume 1 Also Available from Blackwell Publishing: Handbook of Fish Biology and Fisheries Edited by Paul J.B
HANDBOOK OF FISH BIOLOGY AND FISHERIES Volume 1 Also available from Blackwell Publishing: Handbook of Fish Biology and Fisheries Edited by Paul J.B. Hart and John D. Reynolds Volume 2 Fisheries Handbook of Fish Biology and Fisheries VOLUME 1 FISH BIOLOGY EDITED BY Paul J.B. Hart Department of Biology University of Leicester AND John D. Reynolds School of Biological Sciences University of East Anglia © 2002 by Blackwell Science Ltd a Blackwell Publishing company Chapter 8 © British Crown copyright, 1999 BLACKWELL PUBLISHING 350 Main Street, Malden, MA 02148‐5020, USA 108 Cowley Road, Oxford OX4 1JF, UK 550 Swanston Street, Carlton, Victoria 3053, Australia The right of Paul J.B. Hart and John D. Reynolds to be identified as the Authors of the Editorial Material in this Work has been asserted in accordance with the UK Copyright, Designs, and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs, and Patents Act 1988, without the prior permission of the publisher. First published 2002 Reprinted 2004 Library of Congress Cataloging‐in‐Publication Data has been applied for. Volume 1 ISBN 0‐632‐05412‐3 (hbk) Volume 2 ISBN 0‐632‐06482‐X (hbk) 2‐volume set ISBN 0‐632‐06483‐8 A catalogue record for this title is available from the British Library. Set in 9/11.5 pt Trump Mediaeval by SNP Best‐set Typesetter Ltd, Hong Kong Printed and bound in the United Kingdom by TJ International Ltd, Padstow, Cornwall. -

Batoid Wing Skeletal Structure: Novel Morphologies, Mechanical Implications, and Phylogenetic Patterns
JOURNAL OF MORPHOLOGY 264:298–313 (2005) Batoid Wing Skeletal Structure: Novel Morphologies, Mechanical Implications, and Phylogenetic Patterns Justin T. Schaefer* and Adam P. Summers University of California, Irvine, Department of Ecology and Evolutionary Biology, Irvine, California 92697-2525 ABSTRACT The skeleton of the “wings” of skates and oscillatory locomotion. These swimming strategies rays consists of a series of radially oriented cartilaginous can be described by the number of waves (f) moving fin rays emanating from a modified pectoral girdle. Each across the wing during steady swimming (Rosen- fin ray consists of small, laterally oriented skeletal ele- berger, 2001). Oscillators appear to fly through the ments, radials, traditionally represented as simple cylin- water, flapping their wings such that f is less than drical building blocks. High-resolution radiography re- 0.5 at any given time (Heine, 1992). In contrast, veals the pattern of calcification in batoid wing elements, Ͼ and their organization within the fin ray, to be consider- undulators often have many waves (f 1) moving ably more complex and phylogenetically variable than along the wing. Fish that swim with f between 0.5 previously thought. Calcification patterns of radials var- and 1 have been categorized as “semi-oscillatory”. ied between families, as well as within individual pectoral The wing skeleton upon which these locomotor fins. Oscillatory swimmers show structural interconnec- waves are propagated consists of an array of serially tions between fin rays in central areas of the wing. Mor- repeating cartilaginous elements (Fig. 1). The carti- phological variation was strongly predictive of locomotor laginous skeleton of batoids is mineralized to vary- strategy, which we attribute to oscillatory swimmers ing degrees, usually taking the form of a thin layer needing different areas of the wing stiffened than do un- of tiles, tesserae, arranged on the surface of an un- dulatory swimmers. -

Download Full Article 1.2MB .Pdf File
Memoirs of Museum Victoria 74: 379–390 (2016) Published 2016 ISSN 1447-2546 (Print) 1447-2554 (On-line) http://museumvictoria.com.au/about/books-and-journals/journals/memoirs-of-museum-victoria/ Stingray diversification across the end-Cretaceous extinctions TERRY BERTOZZI 1, 2, MICHAEL S.Y. LEE 1, 3, * AND STEPHEN C. DONNELLAN 1, 2 1 South Australian Museum, North Terrace, Adelaide, South Australia 5000, Australia 2 School of Biological Sciences, University of Adelaide, SA 5005, Australia 3 School of Biological Sciences, Flinders University, GPO 2100, Adelaide 5001, Australia * To whom correspondence should be addressed. E-mail: [email protected] Abstract Bertozzi, T., Lee, M.S.Y. and Donnellan, S.C. 2016. Stingray diversification across the end-Cretaceous extinctions. Memoirs of Museum Victoria 74: 379–390. The evolution of stingrays (Myliobatiformes) is assessed using a new phylogeny with near-complete genus-level sampling, and additional molecular data. Stingrays diversified into three primary clades: (A) river stingrays, round rays and typical stingrays, (B) butterfly rays and stingarees and (C) eagle and manta rays. The enigmatic sixgill and deepwater rays (Hexatrygon and Plesiobatis) are not basal stingrays, but are part of the second clade. There is extensive clade-specific variation in molecular evolutionary rates across chondrichthyans: the most appropriate (autocorrelated) divergence dating methods indicate that the extant stingray radiation commenced in the late Cretaceous and continued across the K-Pg boundary. This is highly consistent with the fossil record, and suggests that Cretaceous stingrays, being primarily benthic taxa, were less affected by the K-Pg event than taxa inhabiting the water column.