Module 1: Chemical Basis of Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -

Water Olympics, They Will Each Fold and Float: Need a Copy of the Score Card
WMER OLYMPICS I. Topic Area molecules and the paper fibers is greater than the Properties of water cohesive force between the water molecules. This causes the water molecules to be pulled up the paper towel Il. Introductory Statement against the force of gravity. The attraction between unlike This is a series of four activities that deal with some of molecules is called adhesion. the properties of water. The activities are short and may Bubble Rings: See the background information in the be done one at a time or all together in an “Olympic” “Bubble Busters” activity in this book. format. The activities can be used as an introduction to a water unit with the students discovering some of the VII. ManagementSuggestions properties of water, or they can be used as culminating These four activities may be done as individual lessons activities. Either way, it is important that the children or as centers in an “olympic” format with students rotating discuss the properties of water they have observed after through the activities. The task cards can be run off and doing the activities. placed at each center. Students should be responsible for cleaning up a center before moving on to the next one. III. Math Skills Science Processes An extra supply of paper towels may be placed at each a. Computation a. Observing center to facilitate clean up. It is important that these ac- b. Measuring b. Predicting tivities be followed by class discussions which focus on c. Collecting and recording the water properties involved. data d. Controlling variables VIII. Procedure The procedures for each activity are given on the task IV. -

11 Fluid Statics
CHAPTER 11 | FLUID STATICS 357 11 FLUID STATICS Figure 11.1 The fluid essential to all life has a beauty of its own. It also helps support the weight of this swimmer. (credit: Terren, Wikimedia Commons) Learning Objectives 11.1. What Is a Fluid? • State the common phases of matter. • Explain the physical characteristics of solids, liquids, and gases. • Describe the arrangement of atoms in solids, liquids, and gases. 11.2. Density • Define density. • Calculate the mass of a reservoir from its density. • Compare and contrast the densities of various substances. 11.3. Pressure • Define pressure. • Explain the relationship between pressure and force. • Calculate force given pressure and area. 11.4. Variation of Pressure with Depth in a Fluid • Define pressure in terms of weight. • Explain the variation of pressure with depth in a fluid. • Calculate density given pressure and altitude. 11.5. Pascal’s Principle • Define pressure. • State Pascal’s principle. • Understand applications of Pascal’s principle. • Derive relationships between forces in a hydraulic system. 11.6. Gauge Pressure, Absolute Pressure, and Pressure Measurement • Define gauge pressure and absolute pressure. • Understand the working of aneroid and open-tube barometers. 11.7. Archimedes’ Principle • Define buoyant force. • State Archimedes’ principle. • Understand why objects float or sink. • Understand the relationship between density and Archimedes’ principle. 11.8. Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action • Understand cohesive and adhesive forces. • Define surface tension. • Understand capillary action. 11.9. Pressures in the Body • Explain the concept of pressure the in human body. • Explain systolic and diastolic blood pressures. • Describe pressures in the eye, lungs, spinal column, bladder, and skeletal system. -

Adhesion and Cohesion
Hindawi Publishing Corporation International Journal of Dentistry Volume 2012, Article ID 951324, 8 pages doi:10.1155/2012/951324 Review Article Adhesion and Cohesion J. Anthony von Fraunhofer School of Dentistry, University of Maryland, Baltimore, MD 21201, USA Correspondence should be addressed to J. Anthony von Fraunhofer, [email protected] Received 18 October 2011; Accepted 14 November 2011 Academic Editor: Cornelis H. Pameijer Copyright © 2012 J. Anthony von Fraunhofer. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The phenomena of adhesion and cohesion are reviewed and discussed with particular reference to dentistry. This review considers the forces involved in cohesion and adhesion together with the mechanisms of adhesion and the underlying molecular processes involved in bonding of dissimilar materials. The forces involved in surface tension, surface wetting, chemical adhesion, dispersive adhesion, diffusive adhesion, and mechanical adhesion are reviewed in detail and examples relevant to adhesive dentistry and bonding are given. Substrate surface chemistry and its influence on adhesion, together with the properties of adhesive materials, are evaluated. The underlying mechanisms involved in adhesion failure are covered. The relevance of the adhesion zone and its impor- tance with regard to adhesive dentistry and bonding to enamel and dentin is discussed. 1. Introduction molecular attraction by which the particles of a body are uni- ted throughout the mass. In other words, adhesion is any at- Every clinician has experienced the failure of a restoration, be traction process between dissimilar molecular species, which it loosening of a crown, loss of an anterior Class V restora- have been brought into direct contact such that the adhesive tion, or leakage of a composite restoration. -

Cohesion and Adhesion with Proteins
Cohesion and Adhesion with Proteins Charles R. Frihart Forest Products Laboratory Madison, WI 53726, USA [email protected] Introduction These aggregates can be broken up and may be somewhat uncoiled at pH extremes, especially at high pH conditions, With increasing interest in bio-based adhesives, re- when electrostatic repulsion overcomes hydrophobic at- search on proteins has expanded because historically they traction. Additions of chaotropic agents, some surfactants, have been used by both nature and humans as adhesives. A and certain salts are also used to disaggregate and maybe wide variety of proteins have been used as wood adhe- somewhat uncoil the protein structure. sives. Ancient Egyptians most likely used collagens to bond veneer to wood furniture, then came casein (milk), Table 1. Important reactive components in soy pro- blood, fish scales, and soy adhesives, with soybeans and teins as percent of total protein weight. caseins being important for the development of the ply- Amino Acid Structure % Soy Protein wood and glulam industries. Despite many years of re- - + H2NH2CH2CH2CH2C CH COO Na search on developing adhesive products, it is not clear how Lysine NH2 6.4 the proteins provide good adhesive strength, but some N - + thoughts are expressed here. CH2 CH2 COO Na Histidine N 2.6 H NH2 Protein Structure NH - + Arginine H2N C NHCH2CH2CH2 CH COO Na 7.3 NH2 Understanding protein macromolecular structure is a - + prerequisite for understanding properties because protein Tyrosine HO CH2 COO Na 3.8 structure is so different from that of other adhesives. Ex- NH2 - + cept for structural proteins, such as silk, collagen, and ker- CH2 COO Na Tryptophan 1.4 atin, proteins mainly form globular shapes due to the hy- NH2 N drophobicity of their protein sequences. -
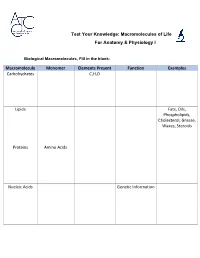
Macromolecules of Life Worksheet
Test Your Knowledge: Macromolecules of Life For Anatomy & Physiology I Biological Macromolecules, Fill in the blank: Macromolecule Monomer Elements Present Function Examples Carbohydrates C,H,O Lipids Fats, Oils, Phospholipids, Cholesterol, Grease, Waxes, Steroids Proteins Amino Acids Nucleic Acids Genetic Information Carbohydrates are classified by __________. The most common simple sugars are glucose, galactose and fructose that are made of a single sugar molecule. These can be classified as __________. Sucrose and __________ are classified as disaccharides; they are made of two monosaccharides joined by a dehydration reaction. The most complex carbohydrates are starch, __________ and cellulose, classified as __________. Lipids most abundant form are __________. Triglycerides building blocks are 1 __________ and 3 __________ per molecule. If a triglyceride only contains __________ bonds that contain the maximum number of __________, then it is classified as a saturated fat. If a triglyceride contains one or more __________ bonds, then it is classified as an unsaturated fat. Lipids are also responsible for a major component of the cell membrane wall that is both attracted to and repelled by water, called __________. The tail of this structure is made of 2 __________, that are water insoluble (hydrophobic). The head of this structure is made of a single __________, that is water soluble (hydrophilic). Proteins building blocks are amino acids that are held together with __________ bonds. These are covalent bonds that link the amino end of one amino acid with the carboxyl end of another. Their overall shape determines their __________. The complex 3D shape of a protein is called a __________. -

Unique Properties of Water!
Name: _______ANSWER KEY_______________ Class: _____ Date: _______________ Unique Properties of Water! Word Bank: Adhesion Evaporation Polar Surface tension Cohesion Freezing Positive Universal solvent Condensation Melting Sublimation Dissolve Negative 1. The electrons are not shared equally between the hydrogen and oxygen atoms of water creating a Polar molecule. 2. The polarity of water allows it to dissolve most substances. Because of this it is referred to as the universal solvent 3. Water molecules stick to other water molecules. This property is called cohesion. 4. Hydrogen bonds form between adjacent water molecules because the positive charged hydrogen end of one water molecule attracts the negative charged oxygen end of another water molecule. 5. Water molecules stick to other materials due to its polar nature. This property is called adhesion. 6. Hydrogen bonds hold water molecules closely together which causes water to have high surface tension. This is why water tends to clump together to form drops rather than spread out into a thin film. 7. Condensation is when water changes from a gas to a liquid. 8. Sublimation is when water changes from a solid directly to a gas. 9. Freezing is when water changes from a liquid to a solid. 10. Melting is when water changes from a solid to a liquid. 11. Evaporation is when water changes from a liquid to a gas. 12. Why does ice float? Water expands as it freezes, so it is LESS DENSE AS A SOLID. 13. What property refers to water molecules resembling magnets? How are these alike? Polar bonds create positive and negative ends of the molecule. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

The Structure and Function of Large Biological Molecules 5
The Structure and Function of Large Biological Molecules 5 Figure 5.1 Why is the structure of a protein important for its function? KEY CONCEPTS The Molecules of Life Given the rich complexity of life on Earth, it might surprise you that the most 5.1 Macromolecules are polymers, built from monomers important large molecules found in all living things—from bacteria to elephants— can be sorted into just four main classes: carbohydrates, lipids, proteins, and nucleic 5.2 Carbohydrates serve as fuel acids. On the molecular scale, members of three of these classes—carbohydrates, and building material proteins, and nucleic acids—are huge and are therefore called macromolecules. 5.3 Lipids are a diverse group of For example, a protein may consist of thousands of atoms that form a molecular hydrophobic molecules colossus with a mass well over 100,000 daltons. Considering the size and complexity 5.4 Proteins include a diversity of of macromolecules, it is noteworthy that biochemists have determined the detailed structures, resulting in a wide structure of so many of them. The image in Figure 5.1 is a molecular model of a range of functions protein called alcohol dehydrogenase, which breaks down alcohol in the body. 5.5 Nucleic acids store, transmit, The architecture of a large biological molecule plays an essential role in its and help express hereditary function. Like water and simple organic molecules, large biological molecules information exhibit unique emergent properties arising from the orderly arrangement of their 5.6 Genomics and proteomics have atoms. In this chapter, we’ll first consider how macromolecules are built. -

SUGGESTED ACTIVITIES (States of Matter)
SUGGESTED ACTIVITIES (States of Matter) From Invitations to Science Inquiry 2nd Edition by Tik L. Liem: Activity Page Number Concept • Can the container hold more? 91 Molecular spacing • The shrinking balloon 92 Molecular spacing • The shrinking mixture of liquids 93 Molecular spacing • The clinging water streams 108 Cohesion • The smaller, the stronger 109 Capillary action • Pour water along a string 110 Adhesion • Where does the cork float? 111 Surface tension • How many pennies can go in? 112 Surface tension • The detergent propelled boat 115 Surface tension From NSF/IERI Science IDEAS Project (See following pages): Activity Page Number Concept • Kids as molecules See following pages Molecular spacing • Poured gas “ “ “ States of Matter • Sticky water “ “ “ Adhesion • The shrinking mixture of liquids w/ measurement Molecular spacing • A”Mazing” Water “ “ “ Cohesion • Cheesecloth Demo “ “ “ Cohesion/Adhesion • Magic Pepper Sinker “ “ “ Cohesion • Merging Streams “ “ “ Cohesion • Pour Water sideways “ “ “ Cohesion/Adhesion • Where does the cork float “ “ “ Surface tension • Magical drops “ “ “ Surface tension • Propel the boat “ “ “ Cohesion From Harcourt Science Teacher’s Ed. Unit E: (For ALL grade levels) Activity Page Number Concept • Solids are smaller E17 (3rd grade text) Molecular spacing NSF/IERI Science IDEAS Project Grant #0228353 CAN THE CONTAINER HOLD MORE? A. Question: How much can a container really hold? B. Materials Needed: 1. A transparent container (glass or plastic). 2. Marbles, sand water and a graduated beaker. C: Procedure: 1. Fill the transparent container up to the brim with marbles. 2. Show the students that you still have sand and water; ask them: “Can I add any other material to this container?” 3. Add sand to the container (shake to settle the sand in between the marbles); ask the same question again.