Hydraulic Traits Are More Diverse in Flowers Than in Leaves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chimonanthus Praecox
Shang et al. Genome Biology (2020) 21:200 https://doi.org/10.1186/s13059-020-02088-y RESEARCH Open Access The chromosome-level wintersweet (Chimonanthus praecox) genome provides insights into floral scent biosynthesis and flowering in winter Junzhong Shang1†, Jingpu Tian1†, Huihui Cheng2†, Qiaomu Yan1, Lai Li1, Abbas Jamal1, Zhongping Xu3,4, Lin Xiang1, Christopher A. Saski5, Shuangxia Jin3,4* , Kaige Zhao1*, Xiuqun Liu1* and Longqing Chen6* * Correspondence: [email protected]. edu.cn; [email protected]; Abstract [email protected]; clqhzau@126. com Background: Wintersweet (Chimonanthus praecox), an important ornamental plant, †Junzhong Shang, Jingpu Tian and has evolved unique fragrant aroma and winter-flowering properties, which are critical Huihui Cheng contributed equally for its successful sexual reproduction. However, the molecular mechanisms underlying to this work. 3National Key Laboratory of Crop these traits are largely unknown in this species. In addition, wintersweet is also a typical Genetic Improvement, Huazhong representative species of the magnoliids, where the phylogenetic position of which Agricultural University, Wuhan, relative to eudicots and monocots has not been conclusively resolved. Hubei 430070, People’s Republic of China Results: Here, we present a chromosome-level wintersweet genome assembly with a 1Key Laboratory of Horticultural total size of 695.36 Mb and a draft genome assembly of Calycanthus chinensis. Plant Biology, Ministry of Education, Huazhong Agricultural University, Phylogenetic analyses of 17 representative angiosperm genomes suggest that Wuhan, Hubei 430070, People’s Magnoliids and eudicots are sister to monocots. Whole-genome duplication Republic of China signatures reveal two major duplication events in the evolutionary history of the 6Southwest Engineering Technology and Research Center of wintersweet genome, with an ancient one shared by Laurales, and a more recent Landscape Architecture, State one shared by the Calycantaceae. -

Annual Benefit Plant Sale 2012
Annual Benefit Plant Sale 2012 Botanic Gardens COLLEGE OF AGRICULTURE & NATURAL RESOURCES Connect to nature Get inspired by wildflowers, naturalistic gardening and meadows in a whole new way with our seasonal garden tours. Enjoy an art class in the garden or learn about native plant gardening, conservation, and habitats by taking one of our classes. And if you can’t visit us, enroll in our new online distance learning program, Mt. Cuba Center Connect. Visit www.mtcubacenter.org to reserve a tour or sign up for a class. Two-Hour Guided Tours | $5 per person Spring Wildflower Tours April 12th – May 27th Summer Twilight Tours May 30th – July 26th 8th Annual Wildflower Celebration |Free th April 29 , 10am – 4pm Purple pitcherplant (Sarracenia purpurea) Greenville, DE P: 302.239.4244 www.mtcubacenter.org INSPIRATION x EDUCATION x CONSERVATION 2 2012 SPRING PLANT SALE CATALOG WEBSITE: http://ag.udel.edu/udbg/events/annualsale.html WELCOME We welcome you to the twentieth annual UDBG benefit plant sale. In addition to its role as the major source of funding for the UDBG, 2012 BENEFIT PLANT SALE CATALOG we hope it also serves as a major educational event for our members and the public. It presents an opportunity to learn about new plants and consider possibilities. We should always look for ways to expand and improve our knowledge about plants and this catalog offers possibilities to accomplish both. As always, we offer an in- depth look at a particular group of plants, this year the genus Camellia. The selection goes beyond offering various cultivars with differing flower color, to a more extensive exploration of the genus with particular emphasis on hardy selections and new hybrids Camellia ‘Autumn Spirit’. -

And Intercontinental Biogeography of Calycanthaceae
Molecular Phylogenetics and Evolution 39 (2006) 1–15 www.elsevier.com/locate/ympev Molecular phylogeny and intra- and intercontinental biogeography of Calycanthaceae Shiliang Zhou a, Susanne S. Renner b, Jun Wen a,c,¤ a Laboratory of Systematic and Evolutionary Botany, Institute of Botany, The Chinese Academy of Sciences, Beijing 100093, China b Department of Biology, Ludwig Maximilians University, Menzinger Str. 67, D-80638 Munich, Germany c Department of Botany, Field Museum of Natural History, 1400 S. Lake Shore Dr., Chicago, IL 60605, USA Received 27 December 2004; revised 27 December 2005; accepted 9 January 2006 Available online 21 February 2006 Abstract Based on nuclear and chloroplast sequences we resolve species relationships in Calycanthaceae and develop a biogeographic hypothe- sis that explains their intercontinental disjunctions and intra-continental diversiWcation in eastern Asia. Fossil-calibrated penalized likeli- hood and Bayesian divergence time estimates indicate that the Northern Hemisphere Calycanthus and Chimonanthus diverged from each other in the mid-Miocene, while the Australian Idiospermum had already diverged by the Upper Cretaceous and likely represents a rem- nant of a former Gondwanan distribution of Calycanthaceae that included South America, as indicated by the occurrence of Cretaceous Calycanthaceae fossils in Brazil. Relationships within Calycanthus were diYcult to resolve, but a shared 155-bp deletion in the trnL–F intergenic spacer unites the two North American species, which were also sisters in a cpDNA restriction site study. Their ancestor appar- ently crossed the Bering land bridge in the Miocene. The six species of Chimonanthus, by contrast, diverged from each other as recently as 1–2 my ago, and a DIVA analysis with four areas of endemism recognized within China suggests three vicariance and two dispersal events within Chimonanthus, with initial vicariance having occurred between eastern and southwestern or central China. -

Evolutionary Directions of Single Nucleotide Substitutions And
Dong et al. BMC Evolutionary Biology (2020) 20:96 https://doi.org/10.1186/s12862-020-01661-0 RESEARCH ARTICLE Open Access Evolutionary directions of single nucleotide substitutions and structural mutations in the chloroplast genomes of the family Calycanthaceae Wenpan Dong1,2, Chao Xu1, Jun Wen1,3 and Shiliang Zhou1,4* Abstract Background: Chloroplast genome sequence data is very useful in studying/addressing the phylogeny of plants at various taxonomic ranks. However, there are no empirical observations on the patterns, directions, and mutation rates, which are the key topics in chloroplast genome evolution. In this study, we used Calycanthaceae as a model to investigate the evolutionary patterns, directions and rates of both nucleotide substitutions and structural mutations at different taxonomic ranks. Results: There were 2861 polymorphic nucleotide sites on the five chloroplast genomes, and 98% of polymorphic sites were biallelic. There was a single-nucleotide substitution bias in chloroplast genomes. A → TorT→ A(2.84%)and G → CorC→ G (3.65%) were found to occur significantly less frequently than the other four transversion mutation types. Synonymous mutations kept balanced pace with nonsynonymous mutations, whereas biased directions appeared between transition and transversion mutations and among transversion mutations. Of the structural mutations, indels and repeats had obvious directions, but microsatellites and inversions were non-directional. Structural mutations increased the single nucleotide mutations rates. The mutation rates per site per year were estimated to be 0.14–0.34 × 10− 9 for nucleotide substitution at different taxonomic ranks, 0.64 × 10− 11 for indels and 1.0 × 10− 11 for repeats. Conclusions: Our direct counts of chloroplast genome evolution events provide raw data for correctly modeling the evolution of sequence data for phylogenetic inferences. -

Calycanthus Chinensis: the Chinese Sweetshrub Jianhua Li ~ Peter Del Tredici
Calycanthus chinensis: The Chinese Sweetshrub jianhua Li ~ Peter Del Tredici chinensis is a ~ beautiful deciduous shrub alycanthuswith a narrow geographic distribution m Zhejiang Province, China. It grows up to ten feet tall with a broad profile. The leaves are oppositely arranged with short petioles and are glossy green with a touch of roughness on the upper surface. In the Boston area its nod- ding flowers appear in mid to late spring. Appearances notwithstand- ing, the sepals and petals are not differentiated (therefore termed tepals): the outer tepals are a silky white with a tinge of pink and a diameter of two to three inches, while the inner tepals are a waxy pale yellow to white with maroon markings. Unlike the native Caly- canthus floridus the flowers are not fragrant and are pollinated by small beetles. Tepals and stamens occupy the rim of a deep floral cup; the ovaries are attached to the side of the cup. The fruits, top-shaped with many seeds, overwinter on the shrub. In its natural habitat, it grows under- neath a canopy and therefore is best cultivated in partial shade with wind protection and good soil mois- ture. In 1998 Michael Dirr described it as "a unique plant but doubtfully " as worthy as Calycanthus floridus. as to the Opinions may vary species’ The pendant flowers of Calycanthus chinensis have an unusual, waxy texture. comparative garden worthiness, but where evolutionary and taxonomic histories are Calycanthus chinensis belongs to Calycan- concerned, C. chinensis definitely provokes more thaceae, which includes two genera and about interest. As a practical matter, the species is rare ten species.’ Chimonanthus (wintersweet) is in the wild and needs our help to survive. -

Effect of Fertilizer Rates & Sources On
UNIVERSITI PUTRA MALAYSIA GROWTH AND NUTRITIONAL QUALITY OF Cosmos caudatus Kunth AS AFFECTED BY RATES OF ORGANIC AND INORGANIC FERTILIZERS AND POST HARVEST COOLING SALUMIAH BINTI MIJIN FP 2014 12 GROWTH AND NUTRITIONAL QUALITY OF Cosmos caudatus Kunth AS AFFECTED BY RATES OF ORGANIC AND INORGANIC FERTILIZERS AND POST HARVEST COOLING UPM SALUMIAH BINTI MIJIN COPYRIGHT MASTER OF SCIENCE © UNIVERSITI PUTRA MALAYSIA 2014 GROWTH AND NUTRITIONAL QUALITY OF Cosmos caudatus Kunth AS AFFECTED BY RATES OF ORGANIC AND INORGANIC FERTILIZERS AND POST HARVEST COOLING UPM By SALUMIAH BINTI MIJIN Thesis Submitted to the School of Graduate Studies, Universiti Putra Malaysia, in Fulfillment of the Requirements for the Degree of Master of COPYRIGHTScience March 2014 © All material contained within the thesis, including without limitation text, logos, icons, photographs and all other artwork, is copyright material of Universiti Putra Malaysia unless otherwise stated. Use may be made of any material contained within the thesis for non-commercial purposes from the copyright holder. Commercial use of material may only be made with the express, prior, written permission of Universiti Putra Malaysia. Copyright © Universiti Putra Malaysia UPM COPYRIGHT © DEDICATION This thesis is dedicated to my beloved husband and son, Abdul Sadar Abuda and Abdul Arieff Anaqiey, who tought me to hope, believe and have faith UPM COPYRIGHT © Abstract of thesis presented to the Senate of Universiti Putra Malaysia in fulfillment of the requirement for the degree of Master of Science GROWTH AND NUTRITIONAL QUALITY OF Cosmos caudatus Kunth AS AFFECTED BY RATES OF ORGANIC AND INORGANIC FERTILIZERS AND POST HARVEST COOLING By SALUMIAH BINTI MIJIN March 2014 Chairman: Associate Professor Siti Aishah Hassan, PhD Faculty: Agriculture UPM A study was conducted to determine the effects of fertilizer sources and rates on growth, physiology and phytonutritional quality of Cosmos caudatus, a herbal species that is normally consumed fresh as a salad. -

Vol. 48 - 2003 Sna Research Conference - Vol
SNA RESEARCH CONFERENCE - VOL. 48 - 2003 SNA RESEARCH CONFERENCE - VOL. 48 - 2003 Introducing 'Venus' Sweetshrub PPAF Thomas G. Ranney and Thomas A. Eaker NC State University, Dept. of Horticultural Science, Mountain Horticultural Crops Research and Extension Center, Fletcher, NC 28732 Index Words: Calycanthus floridus, Calycanthus chinensis, Calycanthus occidentalis, Sinocalycanthus chinensis, ×Sinocalycalycanthus, plant breeding Nature of Work: The sweetshrubs (Calycanthus L./Sinocalycanthus W.C. Cheng and S.Y. Chang) hold great promise as landscape shrubs offering fragrant flowers, attractive foliage, broad adaptability, shade tolerance, and excellent pest resistance. Although the different species and cultivars have merit in their own right, the development of ×Sinocalycalycanthus Lasseigne and Fantz 'Hartlage Wine' demonstrated the potential for further improving sweetshrubs through interspecific/intergeneric hybridization (1). This spectacular hybrid combines many of the best qualities of both parents with large, maroon, wine- red flowers that open widely with a subtle fragrance. 'Hartlage Wine' roots easily from cuttings and once established, plants can be very floriferous and extremely showy. Our recent efforts have focused on developing a wider range of sweetshrub hybrids with a greater diversity of desirable ornamental and commercial characteristics. Results and Discussion: Over the last few years, we have successfully developed additional hybrids between Calycanthus chinensis W.C. Cheng and S.Y. Chang. and Calycanthus floridusL. and new hybrids between Calycanthus chinensis and Calycanthus occidentalis Hook. and Arn. Although most of these hybrids are extremely infertile, we have been able to produce a limited number of advanced generation hybrids that incorporate all three taxa. Many of these hybrids are just now blooming for the first time and the range of characteristics and the potential for new nursery crops is impressive. -

Calycanthus Floridus L. Sweetshrub (Butneria Florida, Buttneria Florida)
Calycanthus floridus L. Sweetshrub (Butneria florida, Buttneria florida) Other Common Names: Carolina Allspice, Carolina Sweetshrub, Common Sweetshrub, Hairy Allspice, Pineapple Shrub, Spicebush, Strawberry Shrub, Sweet Betsy, Sweet Bubby Bush. Family: Calycanthaceae. Cold Hardiness: This species can be grown effectively from USDA hardiness zones 4 to 9. Foliage: Opposite, deciduous, simple, (2) 3 to 5 (6) long by 2 to 3 wide leaves are ovate, elliptic, to obovate; margins are entire, venation pinnate, tips acute, and bases acute to nearly rounded; veins are a lighter green to yellow-green than the medium to dark green blades; leaves are slightly rugose, often lustrous, and mostly glabrous to sparsely hair above, while veins are raised beneath with varying degrees of clear to white tomentose pubescence; leaves are tardily deciduous and can be a good yellow in some years; petioles stout, ½ to ¾ long, pubescent and green or flushed red; foliage is aromatic if bruised. Flower: Fat ovate flower buds open in late spring with numerous petals resemble opening rose buds, then petals reflex to form a broadly cup-shaped 1 to 2 diameter flower; the ovate to lanceolate petals have short hairs on both surfaces; petal colors vary from a purplish pink to dark maroon or rust red-brown; flowers vary from lightly to heavily fragrant, so it is best to purchase in flower to ensure the desired color and fragrance, or purchase a cultivar of known qualities; fragrances are likened to that of a mixture of ripe strawberries, bananas, or pineapples; flowers are axillary or on short branchlets; peduncles are stout and about 1 to 1½ in length. -
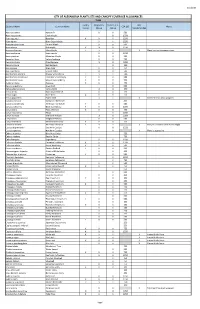
City of Alexandria Plant Lists and Canopy Coverage Allowances Trees
3/1/2019 CITY OF ALEXANDRIA PLANT LISTS AND CANOPY COVERAGE ALLOWANCES TREES Locally Regionally Eastern U.S. Not Botanical Name Common Name CCA (SF) Notes Native Native Native Recommended Abies balsamea Balsam Fir X X 500 Acer leucoderme Chalk Maple X 1250 Acer negundo Boxelder X X X 1250 Acer nigrum Black Sugar Maple X X 1250 Acer pensylvanicum Striped Maple X X 500 Acer rubrum Red maple X X X 1250 Acer saccharinum Silver Maple X X X X Plant has maintenance issues. Acer saccharum Sugar maple X X 1250 Acer spicatum Mountain Maple X X 500 Aesculus flava Yellow Buckeye X X 750 Aesculus glabra Ohio Buckeye X X 1250 Aesculus pavia Red Buckeye X 500 Alnus incana Gray Alder X X 750 Alnus maritima Seaside Alder X X 500 Amelanchier arborea Downy Serviceberry X X X 500 Amelanchier canadensis Canadian Serviceberry X X X 500 Amelanchier laevis Smooth Serviceberry X X X 750 Asimina triloba Pawpaw X X X 750 Betula populifolia Gray Birch X 500 Betula alleghaniensis Yellow Birch X X 750 Betula lenta Black (Sweet) birch X X 750 Betula nigra River birch X X X 750 Betula papyrifera Paper Birch X X X Northern/mountain adapted. Carpinua betulus European Hornbeam 250 Carpinus caroliniana American Hornbeam X X X 500 Carya cordiformis Bitternut Hickory X X X 1250 Carya glabra Pignut Hickory X X X 750 Carya illinoinensis Pecan X 1250 Carya laciniosa Shellbark Hickory X X 1250 Carya ovata Shagbark Hickory X X 500 Carya tomentosa Mockernut Hickory X X X 750 Castanea dentata American Chestnut X X X X Not yet recommended due to Blight Catalpa bignonioides Southern Catalpa X X 1250 Catalpa speciosa Northern Catalpa X X Plant is aggressive. -

Comparative Stem Anatomy of Four Taxa of Calycanthaceae Lindl
View metadata, citation and similar papers at core.ac.uk brought to you by CORE European Journal ISSN 2449-8955 Research Article of Biological Research Comparative stem anatomy of four taxa of Calycanthaceae Lindl. Niroj Paudel, Kweon Heo* Division of Biological Resource Sciences, Kangwon National University, Chuncheon, 24341, South Korea *Corresponding author: Kweon Heo; Phone: +82-33-250-6412; E-mail: [email protected] Received: 15 January 2018; Revised submission: 09 March 2018; Accepted: 14 March 2018 Copyright: © The Author(s) 2018. European Journal of Biological Research © T.M.Karpi ński 2018. This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited. DOI : http://dx.doi.org/10.5281/zenodo.1199578 ABSTRACT able stem anatomical characters are the importance of their function, ontogeny, and phylogeny. The anatomical character is potential value in Calycanthaceae for their taxonomic study. Four Keywords: Anatomical character; Calycanthaceae; species of Calycanthaceae were collected for this Collenchyma; Sclerenchymatous; Vascular bundle. experiment. The experiment was done using the resin methods for preparation of the permanent slide 1. INTRODUCTION for anatomical studies. The anatomical character like two traces of the unilocular vascular bundle, in the Calycanthus, Chimonanthus , and Sinocaly- primary vascular cylinder, contains four cortical canthus are the genus of Calycanthaceae. Sino- vascular bundles in the stem, the unilocular structure calycanthus is native to China . Sinocalycanthus is of primary cylinder, the presence of numerous the synonym of Calycanthus . The literature reveals intercellular space in phloem, the presence of oil cell that long horticulture forms and varieties due to the in the form of scatter in Calycanthus whereas small long cultivation of history. -

2020–2021 Seed Exchange Catalog
MID-ATLANTIC GROUP 2020–2021 Seed Exchange Catalog The 27th annual edition of the Seed Exchange Our Seed Donors Catalog includes 916 seed donations con- Catalog listed seed was generously contributed by our members. tributed by 51 gardeners, from beginners to Where the initial source name is followed by “/”and other member names, the latter identifies those who actually selected, collected, professionals. Approximately 90 new plants cleaned, and then provided descriptions to the members who pre- were donated for the first time. As you can pared the catalog. If a donor reported their zone, you will find it in see, this seed program includes new plants parenthesis. Our sincere thanks to our donors—they make this Seed not previously offered as well as old favorites. Exchange possible. We realize how important this Seed Exchange Bartlett, John 45 (6b) Mahony, Peter 590 (7a) is to HPS and to our members and that is why Bartram’s Garden/ Malarek, David 2608 Katie Jacoby 9975 Malocsay, Jan-Paul 592 (6) we decided to continue the tradition during Berger, Clara 65 McGowan, Brian 3666 (5) the pandemic. Bittmann, Frank 2937 (6a) McShane, Nadeen 627 Bowditch, Margaret 84 (6b) Mills, Michael 2504 We’re sure you’ll enjoy perusing this year’s Boylan, Rebecca 2137 (6b) Nachlas, Sally 2621 (6) selections and you will find plants your gar- Cresson, Charles 199 (7) Norfolk Botanical Staff/ den can’t do without! Since some listed seed Creveling, Beth 200 (7) Julie Finn 1999 (8a) Cyphers, Barry 1181 Perron, William 3321 (6) is in short supply, you are encouraged to Doblmaier, Susan 2515 (6b) Plant Delights 32 place your order early. -

Hydraulic Traits Are More Diverse in Flowers Than in Leaves
DR ADAM RODDY (Orcid ID : 0000-0002-4423-8729) DR CRAIG BRODERSEN (Orcid ID : 0000-0002-0924-2570) Article type : Regular Manuscript Hydraulic traits are more diverse in flowers than in leaves Adam B. Roddy1*, Guo-Feng Jiang2,3, Kunfang Cao2,3, Kevin A. Simonin4, Craig R. Brodersen1 1 School of Forestry & Environmental Studies, Yale University, New Haven, CT, USA 06511 2 State Key Laboratory of Conservation and Utilization of Subtropical Agrobioresources, Guangxi University, Nanning, Guangxi 530004, People’s Republic of China Article 3 Guangxi Key Laboratory of Forest Ecology and Conservation, College of Forestry, Guangxi University, Nanning, Guangxi 530004, People’s Republic of China 4 Department of Biology, San Francisco State University, San Francisco, CA, USA 94132 * Corresponding author: Adam B. Roddy, [email protected]; +1.510.224.4432; Received: 3 December 2018 Accepted: 11 February 2019 ORCID Adam Roddy: 0000-0002-4423-8729 Guofeng Jiang: 0000-0002-3221-8608 Kunfang Cao: 0000-0002-2253-7189 Kevin Simonin: 0000-0002-4990-580X Craig Brodersen: 0000-0002-0924-2570 This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may Accepted lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/nph.15749 This article is protected by copyright. All rights reserved. Summary • Maintaining water balance has been a critical constraint shaping the evolution of leaf form and function. However, flowers, which are heterotrophic and relatively short-lived, may not be constrained by the same physiological and developmental factors.