Chilo Species CP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -

Downloaded from the Same Database
G C A T T A C G G C A T genes Article Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization A. Badji 1,* , D. B. Kwemoi 2, L. Machida 3 , D. Okii 1, N. Mwila 1, S. Agbahoungba 4, F. Kumi 5 , A. Ibanda 1 , A. Bararyenya 1, M. Solemanegy 1, T. Odong 1, P. Wasswa 1, M. Otim 2, G. Asea 2, M. Ochwo-Ssemakula 1, H. Talwana 1, S. Kyamanywa 1 and P. Rubaihayo 1 1 Department of Agricultural Production, Makerere Univesity, P.O. Box 7062 Kampala, Uganda; [email protected] (D.O.); [email protected] (N.M.); [email protected] (A.I.); [email protected] (A.B.); [email protected] (M.S.); [email protected] (T.O.); [email protected] (P.W.); [email protected] (M.O.-S.); [email protected] (H.T.); [email protected] (S.K.); [email protected] (P.R.) 2 Cereals Program, National Crop Resource Research Institute, P.O. Box 7084 Kampala, Uganda; [email protected] (D.B.K.); [email protected] (M.O.); [email protected] (G.A.) 3 Alliance Bioversity International-CIAT, P.O. Box 24384 Kampala, Uganda; [email protected] 4 Laboratory of Applied Ecology, University of Abomey-Calavi, 01BP 526 Cotonou, Benin; [email protected] 5 Department of Crop Science, University of Cape Coast, P.O. Box 5007 PMB Cape Coast, Ghana; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 1 June 2020; Published: 24 June 2020 Abstract: Several species of herbivores feed on maize in field and storage setups, making the development of multiple insect resistance a critical breeding target. -

Hymenoptera: Braconidae) (Indonesian Strain) Against Sugarcane Stalk and Internode Borers
I /Ii,,!. Coli/mi. 15(2): 127-131, 2001 Field evaluation of Cotesiaflavipes Cameron (Hymenoptera: Braconidae) (Indonesian strain) against sugarcane stalk and internode borers R. K. TANWAR and ASHOK VARMA Division of Entomology, Indian Institute of Sugarcane Research Lucknow 226 002, Uttar Pradesh, India E-mail: [email protected] ABS TRA CT: Field trials were conducted on the releases of Cotesia Jlavipes Cameron (Indonesian strain) against sugarcane stalk (Chilo auricilius Dudg.) and internode (Chilo sacchariphagus indiclls Kapur) borers at IISR Farm, Lucknow for consecutive three crop sea sons 1996-97 to 1998-99. The parasitoids were released in one block @ 2000 mated females! ha I month split into four doses from July to October and the other block was treated as check. The results indicated reduction of 56.2, 69.6 and 43.1 per cent in stalk borer infestation in parasitoid released blocks as compared to check, in October during 1996- 97, 1997-98 and 1998~99, respectively. The results remained inconclusive in cases of internode borer due to low infestation. KEY WORDS: Chilo auriciliu.f, Clli/o sacchllriplwgu.f indiclIs. Cotesia Jlavipes. parasitoid. field releases Cotesia Jlavipes Cameron (Hymenoptera: 1979) and Thailand (Suasa-ard and Charernson, Braconidae). an important gregarious larval 1999). In the recent past the Indonesian strain endoparasitoid of different sugarcane borers, has been imported to India through Project namely Chilo infuscatellus Snellen, Chilo Directorate of Biological Control, Bangalore for sacchariphagus indicus Kapur, Chilo evaluation against sugarcane borers. Laboratory tumidicostalis Hmpsn., Sesamia inferens evaluation of this strain has already been done (Walker) and Acigona steniellus (Hmpsn.) is (Tanwar and Varma, 1996). -

Response of Chilo Partellus (Lepidoptera: Crambidae) to Bt Maize in South Africa
Response of Chilo partellus (Lepidoptera: Crambidae) to Bt maize in South Africa J Vorster orcid.org/0000-0001-8126-6860 Dissertation submitted in fulfilment of the requirements for the Masters degree in Environmental Science at the North-West University Supervisor: Prof J van den Berg Co-supervisor: Prof MJ du Plessis Assistant supervisor: Dr A Erasmus Graduation May 2018 23441674 ACKNOWLEDGEMENTS This dissertation would not have been possible without the help of so many people. I am blessed and very grateful to have them in my life. I would like to start with our God Almighty and our Saviour who bestowed upon me the strength, wisdom and peace of mind to finish this project and who also have sent me these blessed people in my life. I would like to thank Prof. Johnnie van den Berg and Dr. Annemie Erasmus for all the guidance and support they have given me. You taught me that small things can make a big difference. Statistics can be difficult sometimes and I thank Prof. Hannalene du Plessis and Prof. Suria Elis for the help with the statistics. Thank you to all the staff at the ARC-GCI that assisted me with the trials in the lab and the planting. Elrine Strydom, Mabel du Toit, Heidi Meyer and Ursula du Plessis, thank you for the countless after hours we had to spend and for the warm hearted kindness you have given me. I would also like to thank my parents whom I dearly love for all the encouragement and motivation to do my best. You taught me that hard work does not come easily, but the fruit that you pick from it is what motivates us. -

An Assessment of Biological Control of the Banana Pseudostem Weevil Odoiporus Longicollis (Olivier) by Entomopathogenic Fungi Beauveria Bassiana T
Biocatalysis and Agricultural Biotechnology 20 (2019) 101262 Contents lists available at ScienceDirect Biocatalysis and Agricultural Biotechnology journal homepage: www.elsevier.com/locate/bab An assessment of biological control of the banana pseudostem weevil Odoiporus longicollis (Olivier) by entomopathogenic fungi Beauveria bassiana T Alagersamy Alagesana, Balakrishnan Padmanabanb, Gunasekaran Tharania, Sundaram Jawahara, Subramanian Manivannana,c,* a PG and Research Department of Biotechnology, Bharath College of Science and Management, Thanjavur, 613 005, Tamil Nadu, India b Division of Crop Protection, National Research Centre for Banana (ICAR), Tiruchirappalli, 620 102, Tamil Nadu, India c Department of Zoology, Kongunadu Arts and Science College, Coimbatore, 641 029, Tamil Nadu, India ARTICLE INFO ABSTRACT Keywords: Banana (Musa sp.) is the most imperative staple food crop for all types of people worldwide, which is commonly Banana production grown in Southeast Asia. Banana plantain can be severely affected by the devastating pest Odoiporus longicollis Odoiporus longicollis that results in severe economic losses in India. Management of weevil pests using chemical methods is harmful to Beauveria bassiana the environment, and cultural methods are also partially successful. Therefore, an alternative approach of plant Bioefficacy defense mediated by endophytic fungi to control banana stem borer larvae is necessary, which could affect the Extracellular enzyme extracellular enzyme chitinase and protease. Among four isolates, Beauveria bassiana isolate KH3 is the most Phylogeny virulent entomopathogenic fungus compared with other isolates, and species identification was achieved using molecular phylogenetic characteristics. The B. bassiana isolate KH3 (1 × 108 conidia/mL-1) is more bioeffective against O. longicollis larvae, causing > 90% significant mortality in 12 and 18 days. -

377 Genus Borbo Evans
14th edition (2015). Genus Borbo Evans, 1949 A catalogue of the Hesperiidae from Europe, Asia and Australia in the British Museum (Natural History): 44, 436 (502 pp.). London. Type-species: Hesperia borbonica Boisduval, by original designation. A predominantly Afrotropical genus containing 23 species. There are 19 Afrotropical species, one of which (borbonica) extends extralimitally. There are a further four extralimital species. *Borbo binga (Evans, 1937) Dark Forest Swift Baoris binga Evans, 1937. A catalogue of the African Hesperiidae indicating the classification and nomenclature adopted in the British Museum: 178 (212 pp.). Type locality: Ivory Coast. Distribution: Ivory Coast, Ghana, Nigeria, Congo, Democratic Republic of Congo. Specific localities: Ivory Coast – Banco (Larsen, 2005a); Lamto (Larsen, 2005a). Ghana – Assin Foso (Maessen, vide Larsen, 2005a); Atewa Range (Belcastro, vide Larsen, 2005a); Kakum National Park (Larsen, 2005a). Nigeria – Ojo near Lagos (Larsen, 2005a). Democratic Republic of Congo – Luali, Mayoumbe district (Ackery et al., 1995). Habitat: Forest (Larsen, 2005a). Habits: A scarce skipper (Larsen, 2005a). Early stages: Nothing published. Larval food: Nothing published. *Borbo borbonica (Boisduval, 1833)# Olive-haired Swift A male Olive-haired Swift (Borbo borbonica) feeding on a Lantana flower Image courtesy Raimund Schutte Hesperia borbonica Boisduval, 1833. Nouvelles Annales du Muséum d’Histoire Naturelle, Paris 2: 213 (149-270). Pamphila borbonica Boisduval. Trimen, 1866a. 1 Pamphila borbonica (Boisduval, 1833). Trimen & Bowker, 1889. Pelopidas borbonica (Boisduval, 1833). Evans, 1937. Pelopidas borbonica Boisduval, 1833. Swanepoel, 1953a. Borbo borbonica (Boisduval, 1833). Dickson & Kroon, 1978. Borbo borbonica (De Boisduval, 1833). Pringle et al., 1994: 335. Borbo borbonica borbonica. Male (Wingspan 42 mm). Left – upperside; right – underside. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -

Downloaded from BOLD Or Requested from Other Authors
www.nature.com/scientificreports OPEN Towards a global DNA barcode reference library for quarantine identifcations of lepidopteran Received: 28 November 2018 Accepted: 5 April 2019 stemborers, with an emphasis on Published: xx xx xxxx sugarcane pests Timothy R. C. Lee 1, Stacey J. Anderson2, Lucy T. T. Tran-Nguyen3, Nader Sallam4, Bruno P. Le Ru5,6, Desmond Conlong7,8, Kevin Powell 9, Andrew Ward10 & Andrew Mitchell1 Lepidopteran stemborers are among the most damaging agricultural pests worldwide, able to reduce crop yields by up to 40%. Sugarcane is the world’s most prolifc crop, and several stemborer species from the families Noctuidae, Tortricidae, Crambidae and Pyralidae attack sugarcane. Australia is currently free of the most damaging stemborers, but biosecurity eforts are hampered by the difculty in morphologically distinguishing stemborer species. Here we assess the utility of DNA barcoding in identifying stemborer pest species. We review the current state of the COI barcode sequence library for sugarcane stemborers, assembling a dataset of 1297 sequences from 64 species. Sequences were from specimens collected and identifed in this study, downloaded from BOLD or requested from other authors. We performed species delimitation analyses to assess species diversity and the efectiveness of barcoding in this group. Seven species exhibited <0.03 K2P interspecifc diversity, indicating that diagnostic barcoding will work well in most of the studied taxa. We identifed 24 instances of identifcation errors in the online database, which has hampered unambiguous stemborer identifcation using barcodes. Instances of very high within-species diversity indicate that nuclear markers (e.g. 18S, 28S) and additional morphological data (genitalia dissection of all lineages) are needed to confrm species boundaries. -

Auwahi Wind Farm Habitat Conservation Plan FY 2018 Annual Report Incidental Take Permit TE64153A-0/ Incidental Take License ITL-17
Auwahi Wind Farm Habitat Conservation Plan FY 2018 Annual Report Incidental Take Permit TE64153A-0/ Incidental Take License ITL-17 Submitted To: Prepared By: 1750 SW Harbor Way, Suite 400 Portland, Oregon 97201 Tel 503-221-8636 Fax 503-227-1287 Auwahi Wind Farm Project Year 6 (FY 2018) Annual Report Table of Contents 1.0 Introduction .......................................................................................................................................................... 1 2.0 Post-Construction Mortality Monitoring ......................................................................................................... 5 2.1 Systematic Carcass Searches ................................................................................................................ 6 2.2 Carcass Persistence Trials .................................................................................................................... 7 2.3 Searcher Efficiency ............................................................................................................................... 8 2.4 Take ......................................................................................................................................................... 9 2.4.1 Direct Take ............................................................................................................................. 9 2.4.2 Indirect Take ......................................................................................................................... 12 2.5 Wildlife -

Evaluation of Some Promising Sugarcane Varieties for Infestation with Two Sugarcane Borers, Yield and Quality Under Different Row Spacing in Luxor Governorate, Egypt
International Journal of Research Studies in Zoology Volume 5, Issue 3, 2019, PP 11-21 ISSN No. 2454-941X DOI: http://dx.doi.org/10.20431/2454-941X.0503002 www.arcjournals.org Evaluation of Some Promising Sugarcane Varieties for Infestation with Two Sugarcane Borers, Yield and Quality under Different Row Spacing in Luxor Governorate, Egypt Fahmy, A.M. 1*, A.S.S. Desoky2, M.O.A. Galal3 1 Plant Protection Dept., Fac. Agri. and Natural Resources, Aswan University, Egypt 2 Plant Protection Dept., Fac. Agri., Sohag University, Egypt. 3Agron. Dept. Sugar Crop Res. Inst., Agric. Res. Cent., Giza, Egypt. *Corresponding Author: Fahmy, A.M., Plant Protection Dept., Fac. Agri. and Natural Resources, Aswan University, Egypt Abstract: The experiment was conducted at El-Mattana Research Station (latitude of 25.25° N and longitude of 32.31° E), Agricultural Research Center, Luxor Governorate, Egypt on a plant cane in 2017/18 and its 1st ratoon in 2018/19 to evaluate the susceptibility of five promising sugarcane varieties viz. G.2004-27, G.84-47, G.2003-47, G.99-103 and C.57-14 compared to the commercial G.T.54-9 variety for infestation with two sugarcane borers (Sesamia cretica and Chilo agamemnon), yield and quality under three inter-row spacing (80, 100 and 120 cm).The studied combinations were randomly distributed in a randomized complete block design in a split-plot distribution, with three replications, where, inter-row spacing were allocated in the main plots, while sugarcane varieties were randomly distributed in the sub-plots. The results indicated that increasing inter-row spacing from 80 to 120 cm led to a significant reduction in dead hearts%, bored stalks%, bored joints, girdled stalks%, mean no. -

Out of the Orient: Post-Tethyan Transoceanic and Trans-Arabian Routes
Systematic Entomology Page 2 of 55 1 1 Out of the Orient: Post-Tethyan transoceanic and trans-Arabian routes 2 fostered the spread of Baorini skippers in the Afrotropics 3 4 Running title: Historical biogeography of Baorini skippers 5 6 Authors: Emmanuel F.A. Toussaint1,2*, Roger Vila3, Masaya Yago4, Hideyuki Chiba5, Andrew 7 D. Warren2, Kwaku Aduse-Poku6,7, Caroline Storer2, Kelly M. Dexter2, Kiyoshi Maruyama8, 8 David J. Lohman6,9,10, Akito Y. Kawahara2 9 10 Affiliations: 11 1 Natural History Museum of Geneva, CP 6434, CH 1211 Geneva 6, Switzerland 12 2 Florida Museum of Natural History, University of Florida, Gainesville, Florida, 32611, U.S.A. 13 3 Institut de Biologia Evolutiva (CSIC-UPF), Passeig Marítim de la Barceloneta, 37, 08003 14 Barcelona, Spain 15 4 The University Museum, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 16 5 B. P. Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii, 96817-0916 U.S.A. 17 6 Biology Department, City College of New York, City University of New York, 160 Convent 18 Avenue, NY 10031, U.S.A. 19 7 Biology Department, University of Richmond, Richmond, Virginia, 23173, USA 20 8 9-7-106 Minami-Ôsawa 5 chome, Hachiôji-shi, Tokyo 192-0364, Japan 21 9 Ph.D. Program in Biology, Graduate Center, City University of New York, 365 Fifth Ave., New 22 York, NY 10016, U.S.A. 23 10 Entomology Section, National Museum of the Philippines, Manila 1000, Philippines 24 25 *To whom correspondence should be addressed: E-mail: [email protected] Page 3 of 55 Systematic Entomology 2 26 27 ABSTRACT 28 The origin of taxa presenting a disjunct distribution between Africa and Asia has puzzled 29 biogeographers for centuries. -
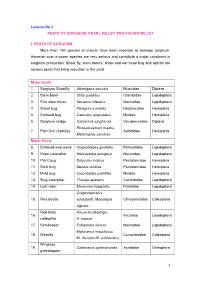
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem.