201605-Weber-Plant-List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

The Campanulaceae of Ohio1
CORE Metadata, citation and similar papers at core.ac.uk Provided by KnowledgeBank at OSU 142 WIENS ET AL. Vol. 62 THE CAMPANULACEAE OF OHIO1 ROBERT W. CRUDEN2 Department of Botany and Plant Pathology, Ohio State University, Columbus 10 In Ohio the family Campanulaceae is represented by three genera: Campanula, Lobelia, and Specularia; and eleven species, of which five are common throughout the state and two are quite limited in their distribution. Following the key to species each species is briefly described, and distribution, common names, chromosome numbers, if known, and other pertinent data are given. Chromosome numbers are those given in Darlington and Wylie (1956) and in the papers of Bowden (1959a, 1959b). Average time of flowering is indi- ^ontribution Nc. 666 of the Department of Botany and Plant Pathology, The Ohio State University. Research completed while a National Science Foundation Co-operative Fellow. 2Present address: Department of Botany, University of California, Berkeley 4, California. THE OHIO JOURNAL OF SCIENCE 62(3): 142, May, 1962. No. 3 CAMPANULACEAE OF OHIO 143 cated as well as the extreme flowering dates as determined from a study of her- barium material. The genera and species are arranged alphabetically. Distri- bution maps are included. A dot represents a collection of a particular species in a given county. No attempt has been made to indicate the general area of collection within the county, as a majority of herbarium specimens do not have this information. It should also be pointed out that many of the collections examined are forty or more years old and thus the distribution maps do not neces- sarily indicate present distribution. -

Literature Cited Robert W. Kiger, Editor This Is a Consolidated List Of
RWKiger 5 Jul 18 Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volume 22, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the abbreviated forms recommended in G. D. R. Bridson and E. R. Smith (1991). Cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix "a"; thus, the sequence of explicit suffixes begins with "b". Some citations in this list have dates suffixed "b" but are not preceded by citations of "[a]" works for the same year, or have dates suffixed "c" but are not preceded by citations of "[a]" and/or "b" works for that year. -

Pruning of Select Native Plants
Pruning of Select Native Plants Courtesy of Jane Gulley, Arkansas Master Gardener Pruning rules of thumb: Shrubs If they bloom once in spring, prune after blooming. Otherwise prune late February or early March. That generally means they bloom on new wood. If they bloom on new wood you can give periodic haircuts for wild branches and it won’t hurt the bush or blooms for next year. Wildflowers Real early spring bloomers leave alone. Many of them are ephemerals and will be gone shortly. For those that are early but not ephemeral, you can cut back the foliage when they look ratty, e.g. Columbine, Amsonia, and Penstemon. If the plant blooms mid-summer you can cut it by half early; when it is 6 inches cut to 3 or 4 inches. If the plant blooms late summer you can give it the early cut PLUS a second cut mid-summer; this might delay flowering a week or so, and some blooms might be a little smaller, but it won’t flop and will be sturdier. The second cut should be about a third, not more than half. Keep records of what you do! Write it down and take a picture so you can review and revise each year. If something doesn’t work, don’t it again. 1. Alexanders, Heart–leaved (Zizia aperta) – trim stems to basal clump after flowering. Remove unwanted seedlings when small. 2. Alexanders, Golden (Zizia aurea) – same as above 3. Amsonia, Arkansas (Amsonia hubrichtii) – shear to half, or more, in spring after flowering; sap causes burns on skin 4. -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -
Manchester Road Redevelopment District: Form-Based Code
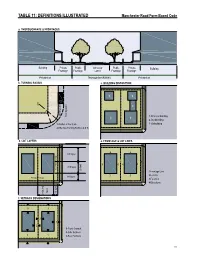
TaBle 11: deFiniTionS illuSTraTed manchester road Form-Based Code a. ThoroughFare & FronTageS Building Private Public Vehicular Public Private Building Frontage Frontage Lanes Frontage Frontage Private lot Thoroughfare (r.o.w.) Private lot b. Turning radiuS c. Building diSPoSiTion 3 3 2 2 1 Parking Lane Moving Lane 1- Principal Building 1 1 2- Backbuilding 1-Radius at the Curb 3- Outbuilding 2-Effective Turning Radius (± 8 ft) d. loT LAYERS e. FronTage & loT lineS 4 3rd layer 4 2 1 4 4 4 3 2nd layer Secondary Frontage 20 feet 1-Frontage Line 2-Lot Line 1st layer 3 3 Principal Frontage 3-Facades 1 1 4-Elevations layer 1st layer 2nd & 3rd & 2nd f. SeTBaCk deSignaTionS 3 3 2 1 2 1-Front Setback 2-Side Setback 1 1 3-Rear Setback 111 Manchester Road Form-Based Code ARTICLE 9. APPENDIX MATERIALS MBG Kemper Center PlantFinder About PlantFinder List of Gardens Visit Gardens Alphabetical List Common Names Search E-Mail Questions Menu Quick Links Home Page Your Plant Search Results Kemper Blog PlantFinder Please Note: The following plants all meet your search criteria. This list is not necessarily a list of recommended plants to grow, however. Please read about each PF Search Manchesterplant. Some may Road be invasive Form-Based in your area or may Code have undesirable characteristics such as above averageTab insect LEor disease 11: problems. NATIVE PLANT LIST Pests Plants of Merit Missouri Native Plant List provided by the Missouri Botanical Garden PlantFinder http://www.mobot.org/gardeninghelp/plantfinder Master Search Search limited to: Missouri Natives Search Tips Scientific Name Scientific Name Common NameCommon Name Height (ft.) ZoneZone GardeningHelp (ft.) Acer negundo box elder 30-50 2-10 Acer rubrum red maple 40-70 3-9 Acer saccharinum silver maple 50-80 3-9 Titles Acer saccharum sugar maple 40-80 3-8 Acer saccharum subsp. -

Complete Iowa Plant Species List
!PLANTCO FLORISTIC QUALITY ASSESSMENT TECHNIQUE: IOWA DATABASE This list has been modified from it's origional version which can be found on the following website: http://www.public.iastate.edu/~herbarium/Cofcons.xls IA CofC SCIENTIFIC NAME COMMON NAME PHYSIOGNOMY W Wet 9 Abies balsamea Balsam fir TREE FACW * ABUTILON THEOPHRASTI Buttonweed A-FORB 4 FACU- 4 Acalypha gracilens Slender three-seeded mercury A-FORB 5 UPL 3 Acalypha ostryifolia Three-seeded mercury A-FORB 5 UPL 6 Acalypha rhomboidea Three-seeded mercury A-FORB 3 FACU 0 Acalypha virginica Three-seeded mercury A-FORB 3 FACU * ACER GINNALA Amur maple TREE 5 UPL 0 Acer negundo Box elder TREE -2 FACW- 5 Acer nigrum Black maple TREE 5 UPL * Acer rubrum Red maple TREE 0 FAC 1 Acer saccharinum Silver maple TREE -3 FACW 5 Acer saccharum Sugar maple TREE 3 FACU 10 Acer spicatum Mountain maple TREE FACU* 0 Achillea millefolium lanulosa Western yarrow P-FORB 3 FACU 10 Aconitum noveboracense Northern wild monkshood P-FORB 8 Acorus calamus Sweetflag P-FORB -5 OBL 7 Actaea pachypoda White baneberry P-FORB 5 UPL 7 Actaea rubra Red baneberry P-FORB 5 UPL 7 Adiantum pedatum Northern maidenhair fern FERN 1 FAC- * ADLUMIA FUNGOSA Allegheny vine B-FORB 5 UPL 10 Adoxa moschatellina Moschatel P-FORB 0 FAC * AEGILOPS CYLINDRICA Goat grass A-GRASS 5 UPL 4 Aesculus glabra Ohio buckeye TREE -1 FAC+ * AESCULUS HIPPOCASTANUM Horse chestnut TREE 5 UPL 10 Agalinis aspera Rough false foxglove A-FORB 5 UPL 10 Agalinis gattingeri Round-stemmed false foxglove A-FORB 5 UPL 8 Agalinis paupercula False foxglove -

How Does Genome Size Affect the Evolution of Pollen Tube Growth Rate, a Haploid Performance Trait?
Manuscript bioRxiv preprint doi: https://doi.org/10.1101/462663; this version postedClick April here18, 2019. to The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv aaccess/download;Manuscript;PTGR.genome.evolution.15April20 license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Effects of genome size on pollen performance 2 3 4 5 How does genome size affect the evolution of pollen tube growth rate, a haploid 6 performance trait? 7 8 9 10 11 John B. Reese1,2 and Joseph H. Williams2 12 Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 13 37996, U.S.A. 14 15 16 17 1Author for correspondence: 18 John B. Reese 19 Tel: 865 974 9371 20 Email: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/462663; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 22 ABSTRACT 23 Premise of the Study – Male gametophytes of most seed plants deliver sperm to eggs via a 24 pollen tube. Pollen tube growth rates (PTGRs) of angiosperms are exceptionally rapid, a pattern 25 attributed to more effective haploid selection under stronger pollen competition. Paradoxically, 26 whole genome duplication (WGD) has been common in angiosperms but rare in gymnosperms. -

Preliminary Checklist of the Terrestrial Flora and Fauna of Fern Cave
Preliminary Checklist of the Terrestrial Flora and Fauna of Fern Cave National Wildlife Refuge ______________________________________________ Prepared for: United States Fish & Wildlife Service Prepared by: J. Kevin England, MAT David Richardson, MS Completed: as of 22 Sep 2019 All rights reserved. Phone: 256-565-4933 Email: [email protected] Flora & Fauna of FCNWR2 ABSTRACT I.) Total Biodiversity Data The main objective of this study was to inventory and document the total biodiversity of terrestrial habitats located at Fern Cave National Wildlife Refuge (FCNWR). Table 1. Total Biodiversity of Fern Cave National Wildlife Refuge, Jackson Co., AL, USA Level of Classification Families Genera Species Lichens and Allied Fungi 14 21 28 Bryophytes (Bryophyta, Anthocerotophyta, Marchantiophyta) 7 9 9 Vascular Plants (Tracheophytes) 76 138 176 Insects (Class Insecta) 9 9 9 Centipedes (Class Chilopoda) 1 1 1 Millipedes (Class Diplopoda) 2 3 3 Amphibians (Class Amphibia) 3 4 5 Reptiles (Class Reptilia) 2 3 3 Birds (Class Aves) 1 1 1 Mammals (Class Mammalia) 2 2 2 Total 117 191 237 II. Vascular Flora (Appendix 3) Methods and Materials To compile a thorough vascular flora survey, several examples of different plant communities at numerous sites were visited and sampled during the study. Approximately 45 minutes was spent documenting community structure at each site. Lastly, all habitats, ecological systems, and plant associations found within the property boundaries were defined based on floristic content, soil characteristics (soil maps) and other abiotic factors. Flora & Fauna of FCNWR3 The most commonly used texts for specimen identification in this study were Flora of North America (1993+), Mohr (1901), Radford et al. -

Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments
U.S. Fish & Wildlife Service Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments Contributors: Printing was made possible through the generous funding from Adkins Arboretum; Baltimore County Department of Environmental Protection and Resource Management; Chesapeake Bay Trust; Irvine Natural Science Center; Maryland Native Plant Society; National Fish and Wildlife Foundation; The Nature Conservancy, Maryland-DC Chapter; U.S. Department of Agriculture, Natural Resource Conservation Service, Cape May Plant Materials Center; and U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Reviewers: species included in this guide were reviewed by the following authorities regarding native range, appropriateness for use in individual states, and availability in the nursery trade: Rodney Bartgis, The Nature Conservancy, West Virginia. Ashton Berdine, The Nature Conservancy, West Virginia. Chris Firestone, Bureau of Forestry, Pennsylvania Department of Conservation and Natural Resources. Chris Frye, State Botanist, Wildlife and Heritage Service, Maryland Department of Natural Resources. Mike Hollins, Sylva Native Nursery & Seed Co. William A. McAvoy, Delaware Natural Heritage Program, Delaware Department of Natural Resources and Environmental Control. Mary Pat Rowan, Landscape Architect, Maryland Native Plant Society. Rod Simmons, Maryland Native Plant Society. Alison Sterling, Wildlife Resources Section, West Virginia Department of Natural Resources. Troy Weldy, Associate Botanist, New York Natural Heritage Program, New York State Department of Environmental Conservation. Graphic Design and Layout: Laurie Hewitt, U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Special thanks to: Volunteer Carole Jelich; Christopher F. Miller, Regional Plant Materials Specialist, Natural Resource Conservation Service; and R. Harrison Weigand, Maryland Department of Natural Resources, Maryland Wildlife and Heritage Division for assistance throughout this project. -

2020 Wholesale Catalog
2020 Wholesale Catalog Production Facilities: Brodhead, WI | Baldwin City, KS 2 | Taylor Creek Restoration Nurseries | www.taylorcreeknurseries.com The highest-quality native, local-genotype plants and seed. With three decades of experience in growing native plants, Taylor Creek Restoration Nurseries is a leader in native plant propagation. With production facilities in southern Wisconsin and eastern Kansas, Taylor Creek supplies the entire Midwest and beyond. Our staff members have degrees in horticulture, reclamation, conservation, and land-use planning. Just as important as the diplomas, our people are passionately dedicated to the promotion and use of native species. 17921 W Smith Rd, PO Box 256 224 E 1260 Rd Brodhead, WI 53520 Baldwin City, KS 66006 P (608) 897-8641 | F (608) 897-2044 P (785) 594-2245 | F (785) 594-2250 E [email protected] E [email protected] TaylorTaylor CreekCreek RestorationRestoration NurseriesNurseries || www.taylorcreeknurseries.comwww.restorationnurseries.com | 3 Philosophy and Mission Taylor Creek Restoration Nurseries is dedicated to the genetic preservation and production of the highest-quality, native, local genotype plants of the Midwest. What are Native, Local-Genotype Species? “Native” merely means the species was natural to the area before European settlement. “Local-genotype” means the strain is adapted to the area in which it is found. This is a very important detail. Just because Panicum virgatum (Switch grass) is native to your area doesn’t mean the genotype of a particular seed is properly “local”. We can probably agree that a genotype from Texas isn’t the same as one from Minnesota – even if the species is the same – and you probably don’t want to plant it in your Minnesota prairie. -

Ozark Ground Flora Response to Landscape-Scale Prescribed Fire
OZARK GROUND FLORA RESPONSE TO LANDSCAPE-SCALE PRESCRIBED FIRE A thesis presented to the faculty of the Graduate School at the University of Missouri-Columbia __________________________________________ In partial fulfillment of the requirements for the degree Masters of Science __________________________________________ by CALVIN JAMES MAGINEL III Dr. Benjamin O. Knapp, Thesis Supervisor JULY 2015 The undersigned, appointed by the dean of the Graduate School, have examined the thesis entitled OZARK GROUND FLORA RESPONSE TO LANDSCAPE-SCALE PRESCRIBED FIRE presented by Calvin James Maginel III, a candidate for the degree of Master of Science and hereby certify that, in their opinion, it is worthy of acceptance. _____________________________________________________ Benjamin O. Knapp, Ph.D. _____________________________________________________ Candace Galen, Ph.D. _____________________________________________________ John M. Kabrick, Ph.D. _____________________________________________________ Rose-Marie Muzika, Ph.D. DEDICATION I dedicate this, the culmination of my ecological and natural education, to my family. My grandparents and parents were constantly encouraging and supporting me when I would drag them off into the woods to look at some new flower, chase fish in creeks for my fish tanks, or sneak up on woodcocks on a cool spring evening. Even when my interests were dragging me all around the country, you were always encouraging me further into the woods. Last, mention of my family would be lacking without including my appreciation of my childhood experiences in woods and stream exploration with my sister. I love you all so much. ACKNOWLEDGEMENTS As this project would not have been possible without the Nature Conservancy and the Missouri Department of Conservation and their generosity with their respective data sets, I thank you many times over.