Ozark Ground Flora Response to Landscape-Scale Prescribed Fire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Early Opinion of an Arkansas Trial Court
University of Arkansas at Little Rock Law Review Volume 5 Issue 3 Article 3 1982 An Early Opinion of an Arkansas Trial Court Morris S. Arnold Follow this and additional works at: https://lawrepository.ualr.edu/lawreview Part of the Courts Commons, and the Legal History Commons Recommended Citation Morris S. Arnold, An Early Opinion of an Arkansas Trial Court, 5 U. ARK. LITTLE ROCK L. REV. 397 (1982). Available at: https://lawrepository.ualr.edu/lawreview/vol5/iss3/3 This Article is brought to you for free and open access by Bowen Law Repository: Scholarship & Archives. It has been accepted for inclusion in University of Arkansas at Little Rock Law Review by an authorized editor of Bowen Law Repository: Scholarship & Archives. For more information, please contact [email protected]. AN EARLY OPINION OF AN ARKANSAS TRIAL COURT Morris S. Arnold* The opinion printed below merits notice because it is appar- ently the oldest surviving opinion of an Arkansas trial judge.' It was delivered in 1824 in a suit in equity, evidently an accounting between partners, which was brought by James Hamilton against William Montgomery in 1823. Relatively little can be discovered about the plaintiff James Hamilton. He was a merchant in Arkansas Post at least as early as November of 1821 when he moved into the "[s]tore lately occupied by Messrs. Johnston [and] Armstrong."2 Montgomery, on the other hand, is quite a well-known character. From 1819 until 1821 he op- erated a tavern at the Post which was an important gathering place:' A muster of the territorial militia was held there on November 25, 1820,4 and the village trustees were elected there in January of the following year.' Moreover, the first regular legislative assembly for the Territory of Arkansas met in February of 1820 in two rooms furnished by Montgomery, perhaps at his tavern. -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

Literature Cited Robert W. Kiger, Editor This Is a Consolidated List Of
RWKiger 5 Jul 18 Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volume 22, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the abbreviated forms recommended in G. D. R. Bridson and E. R. Smith (1991). Cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix "a"; thus, the sequence of explicit suffixes begins with "b". Some citations in this list have dates suffixed "b" but are not preceded by citations of "[a]" works for the same year, or have dates suffixed "c" but are not preceded by citations of "[a]" and/or "b" works for that year. -

Pruning of Select Native Plants
Pruning of Select Native Plants Courtesy of Jane Gulley, Arkansas Master Gardener Pruning rules of thumb: Shrubs If they bloom once in spring, prune after blooming. Otherwise prune late February or early March. That generally means they bloom on new wood. If they bloom on new wood you can give periodic haircuts for wild branches and it won’t hurt the bush or blooms for next year. Wildflowers Real early spring bloomers leave alone. Many of them are ephemerals and will be gone shortly. For those that are early but not ephemeral, you can cut back the foliage when they look ratty, e.g. Columbine, Amsonia, and Penstemon. If the plant blooms mid-summer you can cut it by half early; when it is 6 inches cut to 3 or 4 inches. If the plant blooms late summer you can give it the early cut PLUS a second cut mid-summer; this might delay flowering a week or so, and some blooms might be a little smaller, but it won’t flop and will be sturdier. The second cut should be about a third, not more than half. Keep records of what you do! Write it down and take a picture so you can review and revise each year. If something doesn’t work, don’t it again. 1. Alexanders, Heart–leaved (Zizia aperta) – trim stems to basal clump after flowering. Remove unwanted seedlings when small. 2. Alexanders, Golden (Zizia aurea) – same as above 3. Amsonia, Arkansas (Amsonia hubrichtii) – shear to half, or more, in spring after flowering; sap causes burns on skin 4. -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -
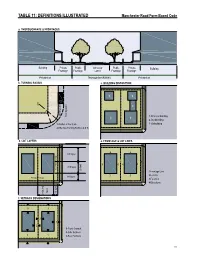
Manchester Road Redevelopment District: Form-Based Code
TaBle 11: deFiniTionS illuSTraTed manchester road Form-Based Code a. ThoroughFare & FronTageS Building Private Public Vehicular Public Private Building Frontage Frontage Lanes Frontage Frontage Private lot Thoroughfare (r.o.w.) Private lot b. Turning radiuS c. Building diSPoSiTion 3 3 2 2 1 Parking Lane Moving Lane 1- Principal Building 1 1 2- Backbuilding 1-Radius at the Curb 3- Outbuilding 2-Effective Turning Radius (± 8 ft) d. loT LAYERS e. FronTage & loT lineS 4 3rd layer 4 2 1 4 4 4 3 2nd layer Secondary Frontage 20 feet 1-Frontage Line 2-Lot Line 1st layer 3 3 Principal Frontage 3-Facades 1 1 4-Elevations layer 1st layer 2nd & 3rd & 2nd f. SeTBaCk deSignaTionS 3 3 2 1 2 1-Front Setback 2-Side Setback 1 1 3-Rear Setback 111 Manchester Road Form-Based Code ARTICLE 9. APPENDIX MATERIALS MBG Kemper Center PlantFinder About PlantFinder List of Gardens Visit Gardens Alphabetical List Common Names Search E-Mail Questions Menu Quick Links Home Page Your Plant Search Results Kemper Blog PlantFinder Please Note: The following plants all meet your search criteria. This list is not necessarily a list of recommended plants to grow, however. Please read about each PF Search Manchesterplant. Some may Road be invasive Form-Based in your area or may Code have undesirable characteristics such as above averageTab insect LEor disease 11: problems. NATIVE PLANT LIST Pests Plants of Merit Missouri Native Plant List provided by the Missouri Botanical Garden PlantFinder http://www.mobot.org/gardeninghelp/plantfinder Master Search Search limited to: Missouri Natives Search Tips Scientific Name Scientific Name Common NameCommon Name Height (ft.) ZoneZone GardeningHelp (ft.) Acer negundo box elder 30-50 2-10 Acer rubrum red maple 40-70 3-9 Acer saccharinum silver maple 50-80 3-9 Titles Acer saccharum sugar maple 40-80 3-8 Acer saccharum subsp. -

History of the U.S. Attorneys
Bicentennial Celebration of the United States Attorneys 1789 - 1989 "The United States Attorney is the representative not of an ordinary party to a controversy, but of a sovereignty whose obligation to govern impartially is as compelling as its obligation to govern at all; and whose interest, therefore, in a criminal prosecution is not that it shall win a case, but that justice shall be done. As such, he is in a peculiar and very definite sense the servant of the law, the twofold aim of which is that guilt shall not escape or innocence suffer. He may prosecute with earnestness and vigor– indeed, he should do so. But, while he may strike hard blows, he is not at liberty to strike foul ones. It is as much his duty to refrain from improper methods calculated to produce a wrongful conviction as it is to use every legitimate means to bring about a just one." QUOTED FROM STATEMENT OF MR. JUSTICE SUTHERLAND, BERGER V. UNITED STATES, 295 U. S. 88 (1935) Note: The information in this document was compiled from historical records maintained by the Offices of the United States Attorneys and by the Department of Justice. Every effort has been made to prepare accurate information. In some instances, this document mentions officials without the “United States Attorney” title, who nevertheless served under federal appointment to enforce the laws of the United States in federal territories prior to statehood and the creation of a federal judicial district. INTRODUCTION In this, the Bicentennial Year of the United States Constitution, the people of America find cause to celebrate the principles formulated at the inception of the nation Alexis de Tocqueville called, “The Great Experiment.” The experiment has worked, and the survival of the Constitution is proof of that. -

Judicial Branch, Pgs. 0231-0338
CHAPTER 5 Judicial Branch “Trick Rider” (Missouri State Archives, Putman Collection) 232 OFFICIAL MANUAL judges throughout the circuit to relieve case load and administrative backlogs. The Supreme Court and court of appeals have general superintending Missouri’s control over all courts and tribunals within their jurisdictions. Original remedial writs may be issued and determined at each level of the court system. Decisions of the Supreme Court are controlling in Judicial all other courts. Selection of Judges System In the first 30 years of Missouri’s statehood, the judges of the supreme, circuit and chancery courts were appointed by the governor with the advice and From its inception in 1820, Missouri state gov- consent of the Senate. After much public discussion, ernment has been constitutionally divided into the Constitution was amended in 1849 to provide three separate branches—the legislative, executive for the popular election of judges, and this system and judicial departments. The judicial department’s remains in effect for most Missouri courts even function is not to make the laws of the state or to today. In most circuits, the judges are elected by the administer them, but to adjudicate the controver- voters in partisan elections. sies that arise between persons and parties, to determine fairly and justly the guilt or innocence of However, in 1940 Missouri voters amended the persons charged with criminal offenses, and to Constitution by adopting the “Nonpartisan Se- interpret the laws of the state as enacted by the leg- lection of Judges Court Plan,” which was placed on islature and carried out by the executive. -

Engineer Cantonment, Missouri Territory, 1819-1820: America's First Biodiversity Ineventory
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Great Plains Research: A Journal of Natural and Social Sciences Great Plains Studies, Center for 2008 Engineer Cantonment, Missouri Territory, 1819-1820: America's First Biodiversity Ineventory Hugh H. Genoways University of Nebraska - Lincoln, [email protected] Brett C. Ratcliffe University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/greatplainsresearch Part of the Other International and Area Studies Commons, Plant Sciences Commons, and the Zoology Commons Genoways, Hugh H. and Ratcliffe, Brett C., "Engineer Cantonment, Missouri Territory, 1819-1820: America's First Biodiversity Ineventory" (2008). Great Plains Research: A Journal of Natural and Social Sciences. 927. https://digitalcommons.unl.edu/greatplainsresearch/927 This Article is brought to you for free and open access by the Great Plains Studies, Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Great Plains Research: A Journal of Natural and Social Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Great Plains Research 18 (Spring 2008):3-31 © 2008 Copyright by the Center for Great Plains Studies, University of Nebraska-Lincoln ENGINEER CANTONMENT, MISSOURI TERRITORY, 1819-1820: AMERICA'S FIRST BIODIVERSITY INVENTORY Hugh H. Genoways and Brett C. Ratcliffe Systematic Research Collections University o/Nebraska State Museum Lincoln, NE 68588-0514 [email protected] and [email protected] ABSTRACT-It is our thesis that members of the Stephen Long Expedition of 1819-20 completed the first biodiversity inventory undertaken in the United States at their winter quarters, Engineer Cantonment, Mis souri Territory, in the modern state of Nebraska. -

Early Government
Chapter 4 Early Government Changing boundaries. Spain protested vigorously the sale of Louisiana, reminding France that Napoleon had given his word that the land would not be sold. In no position to go to war, however, Spain was eventually silent, and the transfer was completed. On December 20, 1803, thirty-year-old William C.C. Claiborne be- came the governor of Louisiana, the largest single territory ever owned by the United States. In an official ceremony in New Orleans, the French flag was lowered as the American flag was raised. Midway, the operators paused and the banners waved side by side momentarily, emphasizing the brotherhood of nations. Seconds later, the Stars and Stripes were flying over New Orleans, and thus over all Louisiana. Some 20,000 non-Indian Americans lived in Louisiana Territory, most of them in or around New Orleans and St. Louis, with a few scattered settlements in between. In March, 1804, Congress passed an act which created two territories in the West — the Territory of New Orleans, south of the 33rd Parallel, and the District of Louisiana, or “Upper Louisiana,” north of it. Temporarily, the District of Louisiana was attached to Indiana Terri- tory, under Governor William Henry Harrison, who became the ninth President of the United States, serving a brief term in 1841. In March, 1805, the district was separated from Indiana Territory and became the Territory of Louisiana. General James Wilkinson, the father of Lieuten- ant James Wilkinson, became governor of the Territory of Louisiana in St. Louis. In 1812, the Territory of New Orleans was admitted to the Union as the state of Louisiana. -

TREATY with the SAUK TREATY with the SAUK 7 Stat
TREATY WITH THE SAUK TREATY WITH THE SAUK 7 Stat. 141, May 13, 1816, Proclaimed December 30, 1816. A treaty of peace and friendship made and concluded at St. Louis between William Clark, Ninian Edwards, and Auguste Chouteau, commissioners plenipotentiary of the United States of America, on the part and behalf of the said states, of the one part, and the undersigned chiefs and warriors of the Sacs of Rock river and the adjacent country, of the other part. WHEREAS by the ninth article of the treaty of peace, which was concluded on the twenty-fourth day of December, eighteen hundred and fourteen, between the United States and Great Britain, at Ghent, and which was ratified by the president, with the advice and consent of the senate, on the seventeenth day of February, eighteen hundred and fifteen, it was stipulated that the said parties should severally put an end to all hostilities with the Indian tribes, with whom they might be at war, at the time of the ratification of said treaty; and to place the said tribes inhabiting their respective territories, on the same footing upon which they stood before the war: Provided, they should agree to desist from all hostilities against the said parties, their citizens or subjects respectively, upon the ratification of the said treaty being notified to them, and should so desist accordingly. And whereas the United States being determined to execute every article of the treaty with perfect good faith, and wishing to be particularly exact in the execution of the article above alluded to, relating to the Indian tribes: The president, in consequence thereof, for that purpose, on the eleventh day of March, eighteen hundred and fifteen, appointed the undersigned William Clark, governor of Missouri territory, Ninian Edwards, governor of Illinois territory, and Auguste Chouteau, esq. -

"Benevolent Plans Meritoriously Applied": How Missouri Almost
“Benevolent Plans Meritoriously Applied:” How Missouri Almost Became an Indian Nation, 1803–1811 BY B. J. MCMAHON Maps such as these were published in the early nineteenth century to plot the general locations of Native American tribes. Such a map as this would have been the best available information for Jefferson. (Image: Cartography Associates) 4 | The Confluence | Spring/Summer 2014 …to carry on the benevolent plans which have been so meritoriously applied “Benevolent Plans Meritoriously Applied:” to the conversion of our aboriginal neighbors from the degradation and wretchedness of savage life to a participation of the improvements of which the How Missouri Almost Became human mind and manners are susceptible in a civilized state. — James Madison, an Indian Nation, 1803–1811 First Inaugural Address, 4 March 18091 In 1803, President Thomas Jefferson designed the first official American governmental policy of relocating Indians, one that encouraged them to become farmers and integrate into the United States as citizens. The Jeffersonian approach to Indian-white relations ostensibly planned for assimilation after the Natives voluntarily relocated to the west. Jefferson and his disciples had differing opinions about the Natives but believed they had the same rights to life, liberty, and property as the whites, and that they expected the United States to uphold honorably all treaties and obligations between them. While not the only advocate of the policy named in his honor, he was the first executive given the power and authority by Congress to treat Native Americans as he saw fit.2 The president envisioned much of the area west of the Mississippi as a land where the Indians could live completely separated from white society east of the river.