Stream Fish Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EDMONTON REGION COURT RESUMPTION PROTOCOL PART 3 Amended: December 1, 2020
EDMONTON REGION COURT RESUMPTION PROTOCOL PART 3 Amended: December 1, 2020 Circuit Point Re-opening Circuit points in the Edmonton Region, with the exception of Ft. Chipewyan and Alexis re-opened in September 2020 for Criminal docket and trial matters. Alexis circuit court will re-open on Nation Land commencing December 3, 2020 and all matters will be heard in this location rather than Mayerthorpe as has previously occurred. Fort Chipewyan will remain closed until further notice and all Criminal Dockets and trials will be conducted remotely unless otherwise directed by the Court. Criminal Court Dockets will run at circuit points, but participants are encouraged to appear remotely with the assistance of duty counsel whenever possible (Duty Counsel 1-855-670-6149). Adjournments by counsel and self represented accused and setting of trials are required to be done pursuant to the CMO Out of Custody Protocol by telephone or email 48 hours prior to the scheduled docket appearance date. Effective immediately all Family and Civil docket matters will be heard remotely (via telephone or Webex) from the Basepoint location. All litigants and Counsel should contact the Clerk's office at the basepoint and ensure that they have a telephone number at which they can be reached on the scheduled court date. If a trial has been set, please contact the clerk for advice as to where the trial will be proceeding. All participants, including counsel, witnesses, and accused persons, are encouraged to contact the base point associated with their circuit point in advance of their scheduled appearance date to confirm that the circuit point is open and operational as intended. -

Fort Saskatchewan- Vegreville
Alberta Provincial Electoral Divisions Fort Saskatchewan- Vegreville Compiled from the 2016 Census of Canada July 2018 Introduction The following report produced by the Office of Statistics and Information presents a statistical profile for the Provincial Electoral Division (PED) of Fort Saskatchewan-Vegreville. A PED is a territorial unit represented by an elected Member to serve in the Alberta Provincial Legislative Assembly. This profile is based on the electoral boundaries that will be in effect for the 2019 Provincial General Election. General characteristics of the PED of Fort Saskatchewan-Vegreville are described with statistics from the 2016 Census of Canada, including: age, sex, marital status, household types, language, Aboriginal identity, citizenship, ethnic origin, place of birth, visible minorities, mobility, dwellings, education, labour force and income. Users are advised to refer to the endnotes of this profile for further information regarding data quality and definitions. Should you have any questions or require additional information, please contact: Ryan Mazan Chief Statistician/Director Office of Statistics and Information Alberta Treasury Board and Finance [email protected] 60 HWY 55 Fort McMurray- 51 Lac La Biche Bonnyville-Cold Lake- Fort Saskatchewan- St. Paul Vegreville 49 !Bonnyville Athabasca-Barrhead- Provincial Electoral Division 62 Westlock HWY 28a Muriel Lake HWY 18 Fort Saskatchewan- 3 WY 2 !H 8 6 Vegreville Smoky Y W Lake Provincial Electoral H St. Paul HWY29 ! Division Elk ! H ! Y 646 !Legal Redwater -
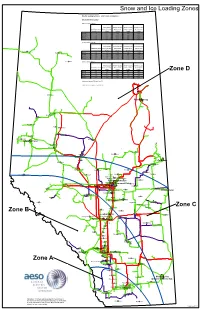
Wet Snow and Wind Loading
Snow and Ice Loading Zones Weather Loading Summary - AESO Tower Development Wet Snow & Wind Loadings 100 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 20 m (Pa) at 30 m (Pa) at 40 m Accretion (mm) Height Height Height Height Zone A 70 77 295 320 340 Zone B 70 71 240 260 280 Zone C 50 67 210 230 245 Zone D 50 64 190 205 220 75 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 20 m (Pa) at 30 m (Pa) at 40 m Accretion (mm) Height Height Height Height Rainbow Lake High Level Zone A 65 75 270 290 310 Zone B 65 70 235 255 270 Zone C 45 65 200 215 230 Zone D 45 62 180 195 210 La Crète 50 Year Return Values Wind Speed Wind Pressure Wind Pressure Wind Pressure Radial Wet Snow (km/hr) at 10m (Pa) at 10 m (Pa) at 20 m (Pa) at 30 m Accretion (mm) Height Height Height Height Zone A 60 74 220 255 280 Zone D Zone B 60 69 190 220 240 Zone C 40 63 160 185 200 Zone D 40 60 145 170 185 Wet snow density 350 kg/m3 at -5C Table Data Last Update: 2010-03-25 Manning Fort McMurray Peace River Grimshaw Fairview Spirit River Falher McLennan High Prairie Sexsmith Beaverlodge Slave Lake Grande Prairie Valleyview Lac la Biche Swan Hills Athabasca Cold Lake Fox Creek Bonnyville Westlock Whitecourt Barrhead Smoky Lake St. -

Transmission Reinforcement in the Central East (Cold Lake, Vegreville and Provost) Area
Transmission Reinforcement in the Central East (Cold Lake, Vegreville and Provost) Area For more information please contact the AESO at 1-888-866-2959, www.aeso.ca or [email protected] Who is the AESO? Alberta’s transmission system, also referred to as the Alberta Interconnected Electric System (AIES), is planned and operated by the Alberta Electric System Operator (AESO). The transmission system is comprised of the high-voltage lines, towers and equipment (generally 69 kV and above) that transmit electricity from generators to lower voltage systems that distribute it to cities, towns, rural areas and large industrial customers. Our job is to maintain safe, reliable and economic operation of the provincial transmission grid. Where is the AESO’s planning study region? The AESO’s planning study region runs from Cold Lake south through the Battle River, Wainwright and Vegreville areas, and east to the Provost area. The Central East region also covers Lloydminster, at the border with Saskatchewan. Larger communities in this region include Cold Lake, Bonnyville, Vermilion, Kitscoty, Lloydminster, St. Paul, Elk Point, Vegreville, Wainwright, Hardisty, Sedgewick, Strome, Jarrow, Edgerton, Castor, and Killarney Lake. Why is transmission development required in the Central East (Cold Lake, Vegreville and Provost) area? Transmission system reinforcement is needed in the study region to meet growing demand for electricity from oil sands development and pipelines, and to interconnect proposed gas fired electricity generation as well as wind farms in the study region. The AESO has received applications to interconnect over 500 megawatts (MW) of wind power and natural gas generation projects in Central East Alberta. -

Science, Assessments and Data Availability Related to Anticipated
Science, Assessments and Data Availability Related to Anticipated Climate and Hydrologic Changes in Inland Freshwaters of the Prairies Region (Lake Winnipeg Drainage Basin) David J. Sauchyn and Jeannine-Marie St. Jacques Fisheries and Oceans Canada Freshwater Institute 501 University Crescent Winnipeg, MB, R3T 2N6 2016 Canadian Manuscript Report of Fisheries and Aquatic Sciences 3107 i Canadian Manuscript Report of Fisheries and Aquatic Sciences Manuscript reports contain scientific and technical information that contributes to existing knowledge but which deals with national or regional problems. Distribution is restricted to institutions or individuals located in particular regions of Canada. However, no restriction is placed on subject matter, and the series reflects the broad interests and policies of Fisheries and Oceans Canada, namely, fisheries and aquatic sciences. Manuscript reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in the data base Aquatic Sciences and Fisheries Abstracts. Manuscript reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Numbers 1-900 in this series were issued as Manuscript Reports (Biological Series) of the Biological Board of Canada, and subsequent to 1937 when the name of the Board was changed by Act of Parliament, as Manuscript Reports (Biological Series) of the Fisheries Research Board of Canada. Numbers 1426 - 1550 were issued as Department of Fisheries and Environment, Fisheries and Marine Service Manuscript Reports. The current series name was changed with report number 1551. Rapport manuscrit canadien des sciences halieutiques et aquatiques Les rapports manuscrits contiennent des renseignements scientifiques et techniques qui constituent une contribution aux connaissances actuelles, mais qui traitent de problèmes nationaux ou régionaux. -

Yellowhead East Health Advisory Council Continues Recruitment for Members
David Thompson/ Yellowhead East Meeting Summary January 22, 2020 / 5:00 p.m. – 8:00 p.m. / Telehealth Introducing your Health Advisory Council members: In attendance David Thompson: Carole Tkach (Chair), Deryl Comeau (Vice Chair), Marie Cornelson, Gerald Johnston, Dawn Konelsky, Phyllis Loewen, Shelagh Slater Regrets: Geraldine Greschner, Melanie Hassett, Peggy Makofka, Selena Redel Yellowhead East: Cyndy Heslin (Chair), Sarah Hissett (Vice Chair), Glenys Reeves, Lesley Binning, Stephanie Munro, Taneen Rudyk Regrets: Deborah McMann, John Erkelenes Alberta Health Services: Sherie Allen, Maya Atallah, Leanne Grant, Debora Okrainetz, Janice Stewart, Marlene Young Community Input Two members of the public were in attendance and shared the following: Community members from Coronation expressed concern about response times of ambulances, as a result of being held up in emergency departments. The feeling is it would be more efficient to have hospital staff assigned to a patient when Emergency Medical Services (EMS) drops them off so ambulances are free to respond to calls. Lara Harries, consultant with Rural Health Professions Action Plan (RhPAP), shared information on the Rhapsody Awards for 2020, and the Building a Better Community Rural Workshop it is hosting. She shared that May 25-29, 2020 is Alberta Rural Health Week and is a great opportunity to host appreciation events/efforts in communities. She encourages attendees to sign up for the monthly and weekly newsletter to be kept up to date on events and news. AHS Presentation Central -

Welcome! Bienvenue!** Vegreville United Church
**Welcome! Bienvenue!** All: Many of us have come from other places, arriving from distant shores, our families arriving years ago or some Vegreville United Church & of us more recently. When settlers came, they were met by Salem United Church others who were already here, already knew these lands, 4th Sunday after Pentecost, June 20, 2021 already lived rich and full lives based on ancient and proud Indigenous Day of Prayer cultures. A Zoom Service. You are invited to say the words to the One: Let us take time to name the peoples of this land now. I prayers and sing the songs, even if you are muted. invite you to speak out loud or in silence the local people(s) who traditionally and still call these lands home. If you are aware of the status of Indigenous claims to this land (unceded, treaty, etc.), REFLECTION please name this too (the people speak the names…). No one can paddle two canoes at the same time. Bantu proverb O God, as we acknowledge the peoples who have lived on and stewarded these lands since time immemorial, and their WE GATHER AS COMMUNITY continued claims to the land, help us to become neighbours that we might live together in better ways. WELCOME All: For we are all kin in Christ, "All My Relations," with ACKNOWLEDGING OUR KINSHIP each other and this earth, its waters, air, animals, and plants. One: Creator, we come together today as diverse, united peoples to give thanks to you, Maker of Heaven and Earth. DANCE WITH THE SPIRIT, CREATION MV #156 Dance with the Spirit early in the mornin’, All: We come to listen, to learn, to sing and pray, to Walk with the Spirit throughout the long day. -

Status of the Arctic Grayling (Thymallus Arcticus) in Alberta
Status of the Arctic Grayling (Thymallus arcticus) in Alberta: Update 2015 Alberta Wildlife Status Report No. 57 (Update 2015) Status of the Arctic Grayling (Thymallus arcticus) in Alberta: Update 2015 Prepared for: Alberta Environment and Parks (AEP) Alberta Conservation Association (ACA) Update prepared by: Christopher L. Cahill Much of the original work contained in the report was prepared by Jordan Walker in 2005. This report has been reviewed, revised, and edited prior to publication. It is an AEP/ACA working document that will be revised and updated periodically. Alberta Wildlife Status Report No. 57 (Update 2015) December 2015 Published By: i i ISBN No. 978-1-4601-3452-8 (On-line Edition) ISSN: 1499-4682 (On-line Edition) Series Editors: Sue Peters and Robin Gutsell Cover illustration: Brian Huffman For copies of this report, visit our web site at: http://aep.alberta.ca/fish-wildlife/species-at-risk/ (click on “Species at Risk Publications & Web Resources”), or http://www.ab-conservation.com/programs/wildlife/projects/alberta-wildlife-status-reports/ (click on “View Alberta Wildlife Status Reports List”) OR Contact: Alberta Government Library 11th Floor, Capital Boulevard Building 10044-108 Street Edmonton AB T5J 5E6 http://www.servicealberta.gov.ab.ca/Library.cfm [email protected] 780-427-2985 This publication may be cited as: Alberta Environment and Parks and Alberta Conservation Association. 2015. Status of the Arctic Grayling (Thymallus arcticus) in Alberta: Update 2015. Alberta Environment and Parks. Alberta Wildlife Status Report No. 57 (Update 2015). Edmonton, AB. 96 pp. ii PREFACE Every five years, Alberta Environment and Parks reviews the general status of wildlife species in Alberta. -

Quaternary Stratigraphy and Surficial Geology Peace River Final Report
SPE 10 Quaternary Stratigraphy and Surficial Geology Peace River Final Report Alberta Energy and Utilities Board Alberta Geological Survey QUATERNARY STRATIGRAPHY AND SURFICIAL GEOLOGY PEACE RIVER - FINAL REPORT Alberta Geological Survey Special Report SPE10 (Canada – Alberta MDA Project M93-04-035) Prepared by L.E. Leslie1 and M.M. Fenton Alberta Energy and Utilities Board Alberta Geological Survey Branch 4th Floor Twin Atria Building 4999 98 Avenue Edmonton, Alberta T6B 2X3 Release date: January 2001 (1)Geo-Environmental Ltd., 169 Harvest Grove Close NE , Calgary AB T3K 4T6 ACKNOWLEDGEMENTS The authors wish to thank Dr. B. Garrett (Geological Survey of Canada) for providing a GSC till standard to use as controls in geochemical analyses of the samples submitted to various laboratories during this study. Thanks also to R. Richardson and R. Olson for reviewing the manuscript and T. Osachuk and D. Troop of Prairie Farm Rehabilitation Administration regarding the Grimshaw groundwater report. The co-operation and assistance received from the personnel at Municipal Districts of 22, 131 and 135, the Peace River Alberta Transportation Regional office is sincerely appreciated. The various companies involved in locating subsurface utilities conducted through Alberta First Call were completed efficiently and without delay The writers are indebted to the willing and able support from the field assistants Solweig Balzer (1993), Nadya Slemko (1994) and Joan Marklund (1995). Special thanks also goes to Dan Magee who drafted the coloured surficial geology map. Partial funding for field support in 1994 was provided by a grant from the Canadian Circumpolar Institute. i PREFACE This report is one of the final products from a project partly funded under the Canada- Alberta Partnership Agreement on Mineral Development (Project M93-04-035) through the Mineral Development Program by what was then called the Alberta Department of Energy (now Department of Resource Development). -

Canada /Dlbsria 45 Northernriverbasins Study
ATHABASCA UNIVERSITY LIBRARY Canada /dlbsria 4 5 3 1510 00168 6063 NorthernRiverBasins Study NORTHERN RIVER BASINS STUDY PROJECT REPORT NO. 105 CONTAMINANTS IN ENVIRONMENTAL SAMPLES: MERCURY IN THE PEACE, ATHABASCA AND SLAVE RIVER BASINS Ft A SH/177/.M45/D675/1996 Contaminants in Donald, David B 168606 DATE DUE BRODART Cat No. 23-221 ; S 8 0 2 , 0 2 / Prepared for the Northern River Basins Study under Project 5312-D1 by David B. Donald, Heather L. Craig and Jim Syrgiannis Environment Canada NORTHERN RIVER BASINS STUDY PROJECT REPORT NO. 105 CONTAMINANTS IN ENVIRONMENTAL SAMPLES: MERCURY IN THE PEACE, ATHABASCA AND SLAVE RIVER BASINS Published by the Northern River Basins Study Edmonton, Alberta ATHABASCA UNIVERSITY March, 1996 OCT 3 1 1996 LIBRARY CANADIAN CATALOGUING IN PUBLICATION DATA Donald, David B. Contaminants in environmental samples: mercury in the Peace, Athabasca and Slave River Basins (Northern River Basins Study project report, ISSN 1192-3571 ; no. 105) Includes bibliographical references. ISBN 0-662-24502-4 Cat. no. R71-49/3-105E 1. Mercury — Environmental aspects -- Alberta -- Athabasca River Watershed. 2. Mercury - Environmental aspects - Peace River Watershed (B.C. and Alta.) 3. Mercury - Environmental aspects -- Slave River Watershed (Alta. And N.W.T.) 4. Fishes - Effect of water pollution on - Alberta -- Athabasca River Watershed. 5. Fishes -- Effect of water pollution on -- Peace River Watershed (B.C. and Alta.) 6. Fishes — Effect of water pollution on — Slave River Watershed (Alta. And N.W.T.) I. Craig, Fleather L. II. Syrgiannis, Jim. III. Northern River Basins Study (Canada) IV. Title. V. Series. SH177.M45D62 1996 363.73'84 C96-980176-9 Copyright© 1996 by the Northern River Basins Study. -

Meyer Adler's Vegreville Store, Ca 1928
Heritage – Yerusha Winter 2015 Adar 5775 VOLUME 17, NO. 2 HERITAGEHERITAGE www.jahsena.ca The Journal of THE JEWISH ARCHIVES & HISTORICAL SOCIETY OF EDMONTON & NORTHERN ALBERTA Inside: Meyer Adler’s Vegreville Store, ca 1928 Archivist’s Report page 2 Rural Beginnings: Part I page 3 Kline Store Report page 10 JAHSENA Archives Follow us on Twitter! Read more about the Adlers’ life in Vegreville on page 5 @Jahsena 2 VISIT OUR WEBSITE: www.jahsena.ca HERITAGE • WINter 2015 hwry From the Archivist’s Desk..., by PAUL GiffoRD HERITAGEHERITAGE The Journal of the Jewish Winter 2015 Archives & Historical Society of Edmonton and Northern Alberta s most of you know, in 2013 we collection, we don’t just collect informa- President Asaw the culmination of a several tion and files from the various organiza- Judy Goldsand years project to open a recreation of tions in Edmonton and its surrounding the HB Kline Jewelry Store along environs; we also collect information Archivist & Editor Fort Edmonton Park’s 1920s Street. on the various family members of our Paul Gifford What you may not be aware of is that community. If you or a relative has a Treasurer JAHSENA provided funding to the family tree, a personal history, some Park to pay for another full time inter- anecdote about a grandparent, even a Howard Davidow preter position through the 2014 season, Bar/Bat Mitzvah invitation, we’d love to Secretary with the understanding that this would preserve it for future generations! To this Hal Simons result in the Kline Store’s categorization end, starting with this edition we will as an A Site, meaning that there would periodically be including a blank family Vice Presidents always be somebody in the Store during tree form; if you’re able and willing to Lawrence Rodnunsky open hours. -

Court File Number 1901-06027 Court of Queen's Bench Of
COURT FILE NUMBER 1901-06027 COURT OF QUEEN’S BENCH OF ALBERTA JUDICIAL CENTRE CALGARY PLAINTIFF ATB FINANCIAL DEFENDANT SOLO LIQUOR STORES LTD., SOLO LIQUOR HOLDINGS LTD., GENCO HOLDINGS LTD., PALI BEDI, JASBIR SINGH HANS, AND TARLOK SINGH TATLA AND IN THE MATTER OF THE RECEIVERSHIP OF SOLO LIQUOR STORES LTD. and SOLO LIQUOR HOLDINGS LTD. APPLICANT FTI CONSULTING CANADA INC. in its capacity as Court-appointed Receiver and Manager of the assets, undertakings and properties of SOLO LIQUOR STORES LTD. and SOLO LIQUOR HOLDINGS LTD. SERVICE LIST Party Telephone Fax Role TORYS LLP 403-776-3744 403-776-3800 Counsel to 525 – 8th Avenue S.W., Receiver 46th Floor Eighth Avenue Place East Calgary, AB T2P 1G1 KYLE KASHUBA Email: [email protected] FTI CONSULTING 403-232-6116 Receiver 520 5th Ave SW Suite 1610 Calgary AB T2P 3R7 DERYCK HELKAA Email: [email protected] 403-454-6041 DUSTIN OLVER Email: [email protected] 403-454-6032 LINDSAY SHIERMAN Email: [email protected] 403-454-6036 27833633.4 Party Telephone Fax Role BLAKE, CASSELS & GRAYDON LLP 403-260-9700 Counsel to ATB 3500, 855 – 2nd Street SW Financial Calgary, AB T2P 4J8 RYAN ZAHARA E-mail: [email protected] 403-260-9628 MATTHEW SUMMERS Email: [email protected] 403-260-9677 ATB FINANCIAL Secured Creditor 3rd Floor, 217 - 16 Avenue NW Calgary, AB T2M 0H5 TRINA HOLLAND Email: [email protected] CROWN CAPITAL PARTNERS INC. Secured Creditor 2730, 333 Bay Street Toronto, ON M5H 2R2 CHRIS JOHNSON Email: [email protected] 416-640-6715 TIM OLDFIELD Email: [email protected] 416-640-6798 McCARTHY TÉTRAULT 403-260-3500 403-260-3501 Counsel to Solo 421 7th Avenue SW Liquor / Solo Suite 4000 Holdings Calgary AB T2P 4K9 WALKER MACLEOD Email: [email protected] 403-260-3710 SEAN COLLINS Email: [email protected] 403-260-3531 MLT AIKINS 306-975-7100 306-975-7145 Counsel to Crown 1500 Saskatoon Square Capital 410 – 22nd Street East Saskatoon, SK S7K 5T6 JEFF LEE, Q.C.