Epipen (Epinephrine Injection, USP) Marketing, MDL No: 2785 Sales Practices and Antitrust Litigation Case No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notice of Proposed Second Administrative Settlement and Request for Public Comment
http://frwebgate3.access.gpo.gov/cgi-bin.. .cID=7723205640+0+0+0&WAISaction=retrieve Superfund Records Confer [Federal Register: September 10, 2002 (Volume 67, Number 175)] ol 11: faff <&&*>! [Notices] BREAK: [Page 57426-57429] OTHER: From the Federal Register Online via GPO Access [wais.access.gpo.gov] [DOCID:frlOse02-81] ENVIRONMENTAL PROTECTION AGENCY [FRL-7373-4] Proposed Second Administrative Cashout Settlement Under Section 122(g) of the Comprehensive Environmental Response, Compensation, and Liability Act; In Re: Beede Waste Oil Superfund Site, Plaistow, NH AGENCY: Environmental Protection Agency. ACTION: Notice of proposed second administrative settlement and request for public comment. [[Page 57427]] SUMMARY: In accordance with section 122(i) of the Comprehensive Environmental Response, Compensation, and Liability Act, as amended, 42 U.S.C. 9622(i), notice is hereby given of a proposed second administrative settlement for recovery of past and projected future response costs concerning the Beeda Waste Oil Superfund Site in Plaistow, New Hampshire with the settling parties listed in the Supplementary Information portion of this notice. The U.S. Environmental Protection Agency--Region I (EPA) is proposing to enter into a second de minimis settlement agreement to address claims under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended (^CERCLA11), 42 U.S.C. 9601 et seq. Notice is being published to inform the public of the proposed second settlement and of the opportunity to comment. This second settlement, embodied in a CERCLA section 122(g) Administrative Order on Consent C'AOC'1), is designed to resolve each settling party's liability at the Site for past work, past response costs and specified future work and response costs through covenants under sections 106 and 107 of CERCLA, 42 U.S.C. -

James Fishkin
James A. Fishkin Partner Washington, D.C. | 1900 K Street, NW, Washington, DC, United States of America 20006-1110 T +1 202 261 3421 | F +1 202 261 3333 [email protected] Services Antitrust/Competition > Merger Clearance > Merger Litigation: U.S. > James A. Fishkin combines both government and private sector experience within his practice, which focuses on mergers and acquisitions covering a wide range of industries, including supermarket chains and other retailers, consumer and food product manufacturers, internet- based firms, chemical and industrial gas firms, and healthcare firms. He has been a key participant in several of the most significant litigated antitrust cases in the last two decades that have set important precedents, including representing Whole Foods Market, Inc. in FTC v. Whole Foods Market, Inc. and the Federal Trade Commission in FTC v. Staples, Inc. and FTC v. H.J. Heinz Co. Mr. Fishkin has also played key roles in securing unconditional clearances for many high-profile mergers, including the merger of OfficeMax/Office Depot and Monster/HotJobs, and approval for other high-profile mergers after obtaining successful settlements, including the merger of Albertsons/Safeway. He also served as the court-appointed Divestiture Trustee on behalf of the Department of Justice in the Grupo Bimbo/Sara Lee bread merger. Mr. Fishkin has been recognized by Chambers USA, The Best Lawyers in America, The Legal 500 , and Benchmark Litigation for his antitrust work. Chambers USA notes that Mr. Fishkin “impresses sources with his ‘very practical perspective,’ with commentators also describing him as ‘very analytical.’” The Legal 500 states that Mr. -
Powerline Plans
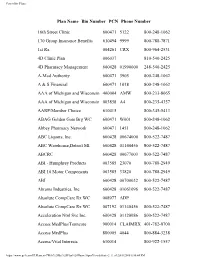
Powerline Plans Plan Name Bin Number PCN Phone Number 16th Street Clinic 600471 5122 800-248-1062 170 Group Insurance Benefits 610494 9999 800-788-7871 1st Rx 004261 CRX 800-964-2531 4D Clinic Plan 006037 810-540-2425 4D Pharmacy Management 600428 01990000 248-540-2425 A-Med Authority 600471 3905 800-248-1062 A & S Financial 600471 1018 800-248-1062 AAA of Michigan and Wisconsin 400004 AMW 800-233-8065 AAA of Michigan and Wisconsin 003858 A4 800-235-4357 AARP/Member Choice 610415 800-345-5413 ABAG Golden Gate Brg WC 600471 W001 800-248-1062 Abbey Pharmacy Network 600471 1451 800-248-1062 ABC Liquors, Inc. 600428 00674000 800-522-7487 ABC Warehouse,Detroit MI 600428 01140456 800-522-7487 ABCRC 600428 00677003 800-522-7487 ABI - Humphrey Products 003585 23070 800-788-2949 ABI 10 Motor Components 003585 33820 800-788-2949 ABI 600428 00700032 800-522-7487 Abrams Industries, Inc. 600428 01061096 800-522-7487 Absolute CompCare Rx WC 008977 ADP Absolute CompCare Rx WC 007192 01140456 800-522-7487 Acceleration Ntnl Svc Inc. 600428 01120086 800-522-7487 Access MedPlus/Tenncare 900014 CLAIMRX 401-782-0700 Access MedPlus 880005 4044 800-884-3238 Access/Vital Interests 610014 800-922-1557 https://www.qs1.com/PLPlans.nsf/Web%20By%20Plan%20Name!OpenView&Start=2 (1 of 2)8/8/2006 6:50:44 PM Powerline Plans Accident Fund of Michigan 600428 00700054 800-522-7487 Accubank Mortgage Inc 600428 00672018 800-522-7487 Aclaim 005848 800-422-5246 ACMG Employers Health Plan 400004 ACM 800-233-8065 Acordia Sr Benefits Horizons 600428 00790015 800-522-7487 https://www.qs1.com/PLPlans.nsf/Web%20By%20Plan%20Name!OpenView&Start=2 (2 of 2)8/8/2006 6:50:44 PM Powerline Plans Plan Name Bin Number PCN Phone Number Acordia Sr Benefits Horizons 600428 00790015 800-522-7487 ACT Industries, Inc. -

Food Marketing Policy Issue Paper
Food Marketing Policy Issue Paper Food Marketing Policy Issue No. 31 November 2002 Papers address particular policy or marketing issues in a non-technical manner. They summarize research Supermarket Prices Need results and provide insights to Come Down for users outside the research community. Single copies are available at no charge. The last page lists all Food Policy Issue Papers to date, and describes other by publication series available from the Food Marketing Ronald W. Cotterill Policy Center. Tel (860) 486-1927 Fax (860) 486-2461 Food Marketing Policy Center email: [email protected] http://www.fmpc.uconn.edu University of Connecticut Food Marketing Policy Center, Department of Agricultural and Resource Economics, University of Connecticut, 1376 Storrs Road, Unit 4021, Storrs, CT 06269-4021 ctnow.com: Supermarket Milk Prices Need To Come Down Page 1 of 2 http://www.ctnow.com/news/opinion/op_ed/hc-milk1126.artnov26.story Supermarket Milk Prices Need To Come Down Ronald W. Cotterill November 26 2002 I surveyed milk prices in 195 grocery stores in southern New England and neighboring areas of New York with four University of Connecticut graduate students two weeks ago. The average price for milk in Connecticut supermarkets was $3.05 per gallon. Yet dairy farmers are suffering because of the lowest prices for their product in 25 years - $1.06 per gallon in October. Adding insult to injury, retail and farm milk prices have been near these levels for a year. What we have is a virulent case of price gouging by supermarkets and, most likely, the dairy processors that supply them. -

Boston Globe Are an Insult To·A-B
City Council At-Large winners· Page 2 Mclaughlin in a Star-t up squeaker ... again Plans for new Star Market District 9 city council seat stays 'Super Store' near with incumbent Brian Mclaughlin Packards Corner moving By Brian Donohue forward District 9 City Councilor Brian McLaughlin is headed Continued on page 23 By Brian Donohue Great 'Acting' job Plan for the construction of a major supennarlcet near Packards Comer in Allston have hit full stride. It's Mayor Menino, now, in a landslide Star Marlcet officials last week presented their plan to construct an 80,000 square foot superstore on Throughout the campaign, Mayor Thomas M. Menino the site ofthe Oste Chevrolet.at 1065 Commonwealth Continued on page 23 Avenue, to the Allston Board of Trade (ABOT), which gave the plan their approval. ABOT President Frank Moy said he felt the plan would have very little, if any, negative effect on the Good buy, Hahnemann neighborhood. and will provide much needed re ...._~tbefonp ofaccess to sbopp.in& as well Mediplex new owner of Hahnemann Hospital as an economic booSt for die ilei~ "We feel the program has a lot of merit. It is By Brian Donohue ~--••reaeede4enctwlnbeabenefit(to) the residents," Moy said. "The onJy other alternative is Purity [Supreme on Hahnemann Hospital Harvard Street in Brookline]," he said. joined the legions of small The Brookline Purity has recently been the target hospitals being purchased by of criticism in that town for the building's state of larger corporations, an disrepair. The chain has promised renovations to the nouncing last week that it exterior of the store, but many Brookline residents has been sold to a major say they are still uncertain about Purity's commit health care corporation The ment to the site. -

Going Places Commencement 2014
2014 l al F BHCC Bunker Hill Community College Magazine Going Places Learn and Earn Internship takes students uptown to new possibilities Commencement 2014 Senator Markey addresses largest graduating class in BHCC history BHCC Turns 40 Old enough for memories, young enough for dreams Features Departments 05 Senator Edward J. Markey Addresses the Largest 05 Campus News Graduating Class in the College’s History 30 Campus Visitors 08 The Learn and Earn Internship Transforms Students’ Lives 34 Off-Campus 16 Q&A with the President 18 Celebrating BHCC's 40th Anniversary 29 Final Exam in a House of Books 32 Annual Gala Inaugurates Ruby Scholarships campus news Bunker Hill Community College In this issue’s sampling of recent BHCC news, President Pam Next Stop: Success personnel transitioning into Board of Trustees Eddinger speaks for community colleges at the White House civilian life, named Bunker As of June 30, 2014 and on NPR, the Boston Private Industry Council (PIC) honors Single Stop director Hill Community College to Marita Rivero three BHCC students, The Boston Foundation recognizes garners recognition the list of top Military Friendly Chair Then and Now Schools. The list singles BHCC’s Single Stop, the Massachusetts Department of The Boston Foundation Antoine Junior Melay* out the colleges, universities Revenue forms a new partnership with the College, and more. recognized Dr. Kathleen Second Vice Chair and trade schools that do the O’Neill with a Change Maker Amy L. Young most to embrace America’s Award for her work as director Secretary military service members, of Bunker Hill Community Viet Phan veterans and spouses as From the College’s Single Stop program. -

Long Range Strategic Plan 1.0 City of Framingham, MA December 30, 2020
Long Range Strategic Plan 1.0 City of Framingham, MA December 30, 2020 Table of Contents INTRODUCTION FROM MAYOR YVONNE M. SPICER 4 EXECUTIVE SUMMARY/OVERVIEW 6 The Five Pillars 6 Five Core Competencies of Municipal Government 8 Organizations of Framingham City Government 8 HISTORY & FRAMINGHAM TODAY 11 THE PUBLIC INPUT PROCESS 12 GOAL STATEMENTS & OBJECTIVES 13 Goal: Conservation & the Environment 13 Goal: Diversity, Equity & Inclusion 14 Goal: Economic Development & Housing 15 Goal: Engagement 16 Goal: Facilities 18 Goal: Finances 19 Goal: Health 20 Goal: Performance 21 Goal: Public Safety 22 Goal: Recreation, History & Culture 23 Goal: Transportation 24 Goal: Workforce 25 2 NEXT STEPS AND IMPLEMENTATION PLAN 26 Implementation Plan 26 Current City Plans 27 ADDENDA 29 I. Survey Text 29 II. Survey Results: Multiple Choice Survey Questions 37 III. Survey Results: Public Comments 49 IV. Responses to Survey Open Response Question 52 Cover Page Graphic: The graphic on the cover of this Plan was generated using the text of the long-form answer comments from the Strategic Plan public input survey. This graphic was produced using WordCloud Generator, a web-based tool that selects and sizes frequently used words and phrases from a given text according to how often the words are used and their relevance. 3 Introduction from Mayor Yvonne M. Spicer Dear Framingham, As Framingham’s first Mayor, working with and hearing from you has been first and foremost on my agenda. Framingham’s first-ever Long Range Strategic Plan is not a document that will sit on a shelf. It is an ongoing opportunity and platform for working together on a Plan that guides our work and vision for the City. -

Food Marketing Review, 1992-93 Sv®«*'
United Sta Department i Agriculture Food Marketing Economic Research Review, 1992-93 Service , 1^ Agricultural Economic Report Number 678 v, <.■.•: 1 ^^ A %^ tsr-f^i,, 'í^/u,'<-v- ^^m\ ''tó's.j öica>*^-,\o<'*f ft ^ »fíríjvl iiv.. m»»? ktrt ':■ es^ ,t»'#*' ft %e* V® A*rz^/.^Ä lt«?i,p\tt*'** -^09 n iWiji«*«SL *oe* 2?^ fOt«^* tt>. »»• ,^tU\V; tA\*« |S9 Gar^^ .ess' cwov e<otitv-^ >»>>^ Sv®«*' fÄ\* CoW SN*" .C^tH c^^tSV^ c^v sv\^o^ oso^ \V). fe* C^'o^Ç!u \i>^¿^^^^ oxtt^ 3ft9 Vct^' \v>. \>sÇt (i^Ö P^ci*<.^G^ %<l^ *»-< \t»- CVl»«* \to- -\Ö9 »■ '::í»rot ^^, .««ftvtssi^- |\\>- It's Easy to Order Another Copy! Just dial 1-800-999-6779. Toll-free in the United States and Canada. Other areas please call 1-703-834-0125. Ask for Food Marketing Review, 1992-93 (AER-678). The cost is $15.00 per copy. For non-U.S. addresses (includes Canada), add 25 percent. Charge your purchases to your VISA or MasterCard, or we can bill you. Or send a check or purchase order (made payable to ERS-NASS) to: ERS-NASS 341 Victory Drive Herndon, VA 22070 We'll fill your order by first-class nrîail. Tha United States Department of Agriculture (USDA) prohibits discrimination in its progmms on the basis of race, color, national origin, sex, religion, age, disability, political beliefe, and marital or familial status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program infonnation (braille, large print, audio tape, etc.) should contact the USDA Office of Communications at (202) 720-5881 (voice) or (202) 720-7808 (TDD). -
Food Marketing Policy Issue Paper
Food Marketing Policy Issue Paper Food Marketing Policy Issue No. 32 November 2002 Papers address particular policy or marketing issues in a non-technical manner. They summarize research Supermarkets, Stop the Gouging - results and provide insights Milk Prices Should Tumble $1 for users outside the research community. Single copies are available at no charge. The last page lists all Food Policy Issue Papers to date, and describes other by publication series available from the Food Marketing Ronald W. Cotterill Policy Center. Tel (860) 486-1927 Fax (860) 486-2461 Food Marketing Policy Center email: [email protected] http://www.fmpc.uconn.edu University of Connecticut Food Marketing Policy Center, Department of Agricultural and Resource Economics, University of Connecticut, 1376 Storrs Road, Unit 4021, Storrs, CT 06269-4021 Projo.com | Providence | Opinion | Contributors Page 1 of 2 Ronald W. Cotterill: Supermarkets, stop the gouging - Milk prices should tumble $1 11/27/2002 STORRS, Conn. WITH THE HELP of four University of Connecticut graduate students, two weeks ago I surveyed milk prices in 195 grocery stores in southern New England and neighboring parts of New York. The average price for milk in Providence supermarkets was $3.03 a gallon (the average prices for the major Providence grocery stores appear in the accompanying table). Currently, dairy farmers are suffering through the lowest prices in 25 years: $1.06 a gallon in October. Adding insult to injury, retail and farm milk prices have been near these levels for a year. According to Massachusetts Department of Agriculture estimates, the cost of processing milk and retailing it in supermarkets is less than $1 a gallon; so retail prices should be $2 or less. -

968-8609 W. Frank Dell II, CMC Is President and Founder of Dell
W. Frank Dell II, CMC 125 Hardesty Road Stamford, CT 06903 (203) 968-8609 W. Frank Dell II, CMC is President and founder of Dellmart & Company, a general management consulting firm. For the last 25 years I have been a consultant to the Food and Consumer Products Industry’s. Prior to forming Dellmart, I was Vice President directing Cresap’s Food and Consumer Products Practice and Senior Partner and Director of Case & Company directing its Food Distribution Practice. I have performed a wide range of assignments in the areas of strategic planning, marketing, supply chain (distribution & logistics), retail operations, pricing, organization design, productivity improvement (warehouse & store), management information systems and market research. Clients have included manufacturers, retailers (food, non-food), grocery wholesalers, foodservice distributors and trade associations in the United States, Canada, Europe, Russia, Africa and the Middle East. I was the creator of Integrated Buying and Inventory System (IBIS) which was a state-of-the-art buying and inventory management computer system. This system integrated turn, promotion and forward buying into a single purchase order. I am a pioneer in the concept and application of Direct Product Profit (DPP) and later Activity Based Costing (ABC). I have directed numerous major studies modeling the costs of manufacturing, retail food, chain drug and food service industries. I have served as an advisor to the Food Marketing Institute, National Mass Retailing Institute, National Candy Wholesaler Association and The Food Business Forum (CIES). Before entering consulting I was Manager of Forecasting & Administration for Colgate-Palmolive Company. Prior corporate experience includes positions with American Can and General Foods. -

United States of America Before Federal Trade Commission
Bl92106 9510086 UNITED STATES OF AMERICA BEFORE FEDERAL TRADE COMMISSION ) In the Matter of ) ) THE STOP & SHOP COMPANIES, INC., ) a corporation; and ) Docket No. C-3649 ) SSC ASSOCIATES, L.P., ) a limited partnership. ) __________________________________ ) COMPLAINT Pur suant to the prov isions of the Federal Trad e Commi ssion Act , a nd by v irtue o f the autho rity v ested i n it by said Act, the Federal Trade Commission ( 11 Commission 11 ), hav ing reas on to b e lieve that respondents, The Stop . & Shop Companies, Inc. ( 11 Stop & Shop"), a corporation, and ' ssc Associates, L . P . (" SSC Associates"), a limited partnership, both s ubject to the jurisdiction of the Commission, have entered into an agreement to 11 acquire Purity Supreme , Inc. ("Purity ) in violation of Section 7 o f the Clayton Act, as amended, 15 U. S.C. § 1 8 , and Sect i o n 5 o f the Federal Trade Commission Act, as amended, 15 U . S.C. § 45 , a nd that a proceeding in respect thereof would be in the public interest, hereby issue s its complaint, stating its charges as follows : Defi nit i o ns 1. For the purposes of this c omplaint: 11 Supermarket 11 means a full-line retail grocery sto re with annual sales of at least two million do llars that carries a wide variety of food and grocery items in particular produ~t categories, including bread and dairy products; refrige rated and fro zen food and beverage products; fresh and prepared meats and poultry; produce , including fresh fruits and vegetables; shelf-stable food and beverage product s, including canned and other types o f packaged produc ts; staple foodstuffs, which may incl ude salt, sugar, flour, sauces, spices, coffee, and tea; and o the r grocery products, including nonfood items such as soaps, detergents, paper go ods, o ther ho useho ld products, and health and beauty aids . -

Preliminary Draft No. 9 (September 4, 2012)
Restatement of the Law Third Employment Law Preliminary Draft No. 9 (September 4, 2012) SUBJECTS COVERED CHAPTER 4 Principles of Employer Liability for Harm to Employees (revised) CHAPTER 7 Employee Privacy and Autonomy (revised) TOPIC 2 Protection of Employee Personal Autonomy CHAPTER 9 Remedies (black letter and Comments only) APPENDIX Black Letter of Preliminary Draft No. 9 The Executive Office The American Law Institute 4025 Chestnut Street Philadelphia, PA 19104-3099 Telephone: (215) 243-1626 • Fax: (215) 243-1636 E-mail: [email protected] • Website: http://www.ali.org ©2012 by The American Law Institute All Rights Reserved This document is submitted to the Advisers for their meeting on September 21 (at 9:00 a.m.), 2012, and to the Members Consultative Group for their meeting on September 22 (at 10:00 a.m.), 2012, both meetings at ALI Headquarters, 4025 Chestnut Street, Philadelphia, Pennsylvania. As of the date it was printed, it had not been considered by the Council or membership of The American Law Institute, and therefore does not represent the position of the Institute on any of the issues with which it deals. © 2012 by The American Law Institute Preliminary Draft – Not approved The American Law Institute Roberta Cooper Ramo, President Allen D. Black, 1st Vice President Douglas Laycock, 2nd Vice President Carolyn Dineen King, Treasurer Susan Frelich Appleton, Secretary Lance Liebman, Director Stephanie A. Middleton, Deputy Director COUNCIL Kenneth S. Abraham, University of Virginia School of Law, Charlottesville, VA Susan Frelich Appleton, Washington University School of Law, St. Louis, MO Kim J. Askew, K&L Gates, Dallas, TX José I.