The Nature of Tactile Agnosia: a Case Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cognitive Emotional Sequelae Post Stroke
11/26/2019 The Neuropsychology of Objectives 1. Identify various cognitive sequelae that may result from stroke Stroke: Cognitive & 2. Explain how stroke may impact emotional functioning, both acutely and long-term Emotional Sequelae COX HEALTH STROKE CONFERENCE BRITTANY ALLEN, PHD, ABPP, MBA 12/13/2019 Epidemiology of Stroke Stroke Statistics • > 795,000 people in the United States have a stroke • 5th leading cause of death for Americans • ~610,000 are first or new strokes • Risk of having a first stroke is nearly twice as high for blacks as whites • ~1/4 occur in people with a history of prior stroke • Blacks have the highest rate of death due to stroke • ~140,000 Americans die every year due to stroke • Death rates have declined for all races/ethnicities for decades except that Hispanics have seen • Approximately 87% of all strokes are ischemic an increase in death rates since 2013 • Costs the United States an estimated $34 billion annually • Risk for stroke increases with age, but 34% of people hospitalized for stroke were < 65 years of • Health care services age • Medicines to treat stroke • Women have a lower stroke risk until late in life when the association reverses • Missed days of work • Approximately 15% of strokes are heralded by a TIA • Leading cause of long-term disability • Reduces mobility in > 50% of stroke survivors > 65 years of age Source: Centers for Disease Control Stroke Death Rates Neuropsychological Assessment • Task Engagement • Memory • Language • Visuospatial Functioning • Attention/Concentration • Executive -

Prosopagnosia by B
J. Neurol. Neurosurg. Psychiat., 1959, 22, 124. PROSOPAGNOSIA BY B. BORNSTEIN and D. P. KIDRON From the Department of Neurology, Beilinson Hospital, Petah Tiqva, Israel "And what is the nature of this knowledge or recollection? I mean to ask, Whether a person, who having seen or heard or in any way perceived anything, knows not only that, but has a conception of something else which is the subject, not of the same but of some other kind of knowledge, may not be fairly said to recollect that of which he has the conception?" "And when the recollection is derived from like things, then another consideration is sure to arise, which is, Whether the likeness in any degree falls short or not of that which is recollected?" "The Philosophy of Plato " Phaedo (the Jowett translation). Does visual agnosia exist in a partial or isolated bances in sensation time, in adaptation time, in form, in which certain qualities only are affected, visual acuity, and in brightness discrimination. as opposed to generalized visual agnosia? Many Ettlinger (1956) rejected Bay's contentions. After workers cast doubt on this concept, maintaining analysing 30 cases of head injury, he showed that that partial visual agnosia is no more than a com- some patients had neither field nor perceptual bination of defects in vision, memory, and orienta- defects, others had field but not perceptual defects, tion, appearing together. and only in eight of the 30 patients were field and The clinical elucidation of partial visual agnosia perceptual defects found together. It is true that is likely to be affected by the patient's intellectual visual agnosia is frequently associated with homony- capacity, his mental state at the time of examination, mous hemianopsia, but despite this there are cases and his ability to cooperate without being influenced of hemianopsia without gnostic defects. -
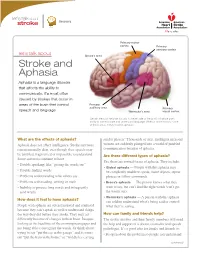
Stroke and Aphasia Aphasia Is a Language Disorder That Affects the Ability to Communicate
Recovery Primary motor cortex Primary sensory cortex let’s talk about Broca’s area Stroke and Aphasia Aphasia is a language disorder that affects the ability to communicate. It’s most often caused by strokes that occur in areas of the brain that control Primary auditory area Primary speech and language. Wernicke’s area visual cortex Certain areas of the brain (usually in the left side of the brain) influence one’s ability to communicate and understand language. When a stroke occurs in one of these areas, it may result in aphasia. What are the effects of aphasia? sender plissen.” Thousands of alert, intelligent men and Aphasia does not affect intelligence. Stroke survivors women are suddenly plunged into a world of jumbled remain mentally alert, even though their speech may communication because of aphasia. be jumbled, fragmented or impossible to understand. Are there different types of aphasia? Some survivors continue to have: Yes, there are several forms of aphasia. They include: • Trouble speaking, like “getting the words out” • Global aphasia — People with this aphasia may • Trouble finding words be completely unable to speak, name objects, repeat • Problems understanding what others say phrases or follow commands. • Problems with reading, writing or math • Broca’s aphasia — The person knows what they • Inability to process long words and infrequently want to say, but can’t find the right words (can’t get used words the words out). • Wernicke’s aphasia — A person with this aphasia How does it feel to have aphasia? can seldom understand what’s being said or control People with aphasia are often frustrated and confused what they’re saying. -

Can We Lose Memories of Faces? Content Specificity and Awareness in a Prosopagnosic
Can We Lose Memories of Faces? Content Specificity and Awareness in a Prosopagnosic Nancy L. Etcoff Department of Brain and Cognitive Sciences Massachusetts Institute of Technology Neuropsychology Laboratory Massachusetts General Hospital Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/3/1/25/1755723/jocn.1991.3.1.25.pdf by guest on 18 May 2021 Roy Freeman Division of Neurology New England Deaconess Hospital Beth Israel Hospital Harvard Medical School Kyle R. Cave Department of Psychology University of California, San Diego Abstract H Prosopagnosia is a neurological syndrome in which patients nonfacial channels. The only other categories of shapes that he cannot recognize faces. Kecently it has been shown that some has marked trouble recognizing are animals and emotional prosopagnosics give evidence of “covert” recognition: they expressions, though even these impairments were not as severe show greater autonomic responses to familiar faces than to as the one for faces. Three measures (sympathetic skin re- unfamiliar ones, and respond differently to familiar faces in sponse, pupil dilation, and learning correct and incorrect learning and interference tasks. Although some patients do not names of faces) failed to show any signs of covert face recog- show covert recognition, this has usually been attributed to an nition in LH, though the measures were sensitive enough to “apperceptive” deficit that impairs perceptual analysis of the reflect autonomic reactions in LH to stimuli other than faces, input. The implication is that prosopagnosia is a deficit in access and face familiarity in normal controls. Thus prosopagnosia to, or awareness of, memories of faces: the inducing brain cannot always be attributed to a mere absence of awareness injury does not destroy the memories themselves. -

Abadie's Sign Abadie's Sign Is the Absence Or Diminution of Pain Sensation When Exerting Deep Pressure on the Achilles Tendo
A.qxd 9/29/05 04:02 PM Page 1 A Abadie’s Sign Abadie’s sign is the absence or diminution of pain sensation when exerting deep pressure on the Achilles tendon by squeezing. This is a frequent finding in the tabes dorsalis variant of neurosyphilis (i.e., with dorsal column disease). Cross References Argyll Robertson pupil Abdominal Paradox - see PARADOXICAL BREATHING Abdominal Reflexes Both superficial and deep abdominal reflexes are described, of which the superficial (cutaneous) reflexes are the more commonly tested in clinical practice. A wooden stick or pin is used to scratch the abdomi- nal wall, from the flank to the midline, parallel to the line of the der- matomal strips, in upper (supraumbilical), middle (umbilical), and lower (infraumbilical) areas. The maneuver is best performed at the end of expiration when the abdominal muscles are relaxed, since the reflexes may be lost with muscle tensing; to avoid this, patients should lie supine with their arms by their sides. Superficial abdominal reflexes are lost in a number of circum- stances: normal old age obesity after abdominal surgery after multiple pregnancies in acute abdominal disorders (Rosenbach’s sign). However, absence of all superficial abdominal reflexes may be of localizing value for corticospinal pathway damage (upper motor neu- rone lesions) above T6. Lesions at or below T10 lead to selective loss of the lower reflexes with the upper and middle reflexes intact, in which case Beevor’s sign may also be present. All abdominal reflexes are preserved with lesions below T12. Abdominal reflexes are said to be lost early in multiple sclerosis, but late in motor neurone disease, an observation of possible clinical use, particularly when differentiating the primary lateral sclerosis vari- ant of motor neurone disease from multiple sclerosis. -

THE CLINICAL ASSESSMENT of the PATIENT with EARLY DEMENTIA S Cooper, J D W Greene V15
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2005.081133 on 16 November 2005. Downloaded from THE CLINICAL ASSESSMENT OF THE PATIENT WITH EARLY DEMENTIA S Cooper, J D W Greene v15 J Neurol Neurosurg Psychiatry 2005;76(Suppl V):v15–v24. doi: 10.1136/jnnp.2005.081133 ementia is a clinical state characterised by a loss of function in at least two cognitive domains. When making a diagnosis of dementia, features to look for include memory Dimpairment and at least one of the following: aphasia, apraxia, agnosia and/or disturbances in executive functioning. To be significant the impairments should be severe enough to cause problems with social and occupational functioning and the decline must have occurred from a previously higher level. It is important to exclude delirium when considering such a diagnosis. When approaching the patient with a possible dementia, taking a careful history is paramount. Clues to the nature and aetiology of the disorder are often found following careful consultation with the patient and carer. A focused cognitive and physical examination is useful and the presence of specific features may aid in diagnosis. Certain investigations are mandatory and additional tests are recommended if the history and examination indicate particular aetiologies. It is useful when assessing a patient with cognitive impairment in the clinic to consider the following straightforward questions: c Is the patient demented? c If so, does the loss of function conform to a characteristic pattern? c Does the pattern of dementia conform to a particular pattern? c What is the likely disease process responsible for the dementia? An understanding of cognitive function and its anatomical correlates is necessary in order to ascertain which brain areas are affected. -

Prosopagnosia: a Clinical, Psychological, and Anatomical Study of Three Patients
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.40.4.395 on 1 April 1977. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1977, 40, 395-403 Prosopagnosia: a clinical, psychological, and anatomical study of three patients A. M. WHITELEY' AND ELIZABETH K. WARRINGTON From the Department ofNeurology, The London Hospital, and the Department ofPsychology, National Hospital, Queen Square, London SUMMARY Three patients with prosopagnosia are described of whom two had right occipital lesions. An analysis of visual and perceptual functions demonstrated a defect in perceptual classi- fication which appeared to be stimulus-specific. A special mechanism for facial recognition is postu- lated, and the importance of the right sided posterior lesion is stressed. Prosopagnosia is a rare but interesting condition unreliably, as pointers to cerebral lesions, and most in which recognition of faces is impaired. The cases have a left homonymous defect indicating right sufferer is quite unable to identify people purely by hemisphere disease, but not excluding a left sided their facial appearance but can do so without lesion (Meadows, 1974a). There are many cases, Protected by copyright. difficulty by their voice and by visual clues such as however, with bilateral field defects indicating clothing, hair colour, and gait. Recognition of other bilateral lesions, but there are cases with right visual material can be intact, but in some cases highly homonymous defects and cases with no field defects discriminative visual skills, such as species of birds at all. There are several case reports where surgery and types of fruit, are impaired (Bornstein, 1963; to right temporal and occipital lobes is responsible, De Renzi et al., 1968). -
• Classifications of Aphasia Expressive Vs. Receptive Fluent Vs
12/7/2018 APHASIA Aphasia is an acquired communication disorder that impairs a person’s ability to process LANGUAGE, but DOES NOT AFFECT intelligence. Aphasia impairs the ability to speak and understand others. -National Aphasia Association LANGUAGE Language is a system of communication that uses symbolism. K L U $ + M – Phonemes: perceptually distinct unit of sounds Words: sounds combined & given meaning Sentences: combination of syntax (rules) and semantics (meaning). • CLASSIFICATIONS OF APHASIA EXPRESSIVE VS. RECEPTIVE FLUENT VS. NON- FLUENT 1 12/7/2018 -NATIONAL APHASIA ASSOCIATION -COURTESY OF MY-MS.ORG MCA DISTRIBUTION -SLIDESHARE.NET 2 12/7/2018 BROCA’S APHASIA * short utterances * limited vocabulary * halting, effortful speech *mild comprehension deficits Lesion * Inferior frontal gyrus Choose Sentence Speech Coordinate Speak Idea Words Structure Sounds Articulate Pragmatics Muscles Fluently (Semantics) (Syntax) (Phonology) SAMPLE OF BROCA’S THERAPY FROM TACTUS THERAPY 3 12/7/2018 WERNICKE’S APHASIA • Comprehension is poor (auditory & reading) • Fluent, intact prosody • Logorrhea, press of speech • Neologisms, Paraphasias • Lack of awareness Lesion Temporo-Parietal, Posterior section of the superior temporal gyrus near the auditory cortex Auditory Preparation Attach Input Perception Recognition Phonological For Meaning Analysis Output WERNICKE’S APHASIA FROM TACTUS THERAPY 4 12/7/2018 GLOBAL APHASIA * severe language deficit * responds to personally relevant language * responds to non-verbal cues * some automatic speech Lesion -

THE SYNDROMES of the ARTERIES of the BRAIN and SPINAL CORD Part 1 by LESLIE G
65 Postgrad Med J: first published as 10.1136/pgmj.29.328.65 on 1 February 1953. Downloaded from THE SYNDROMES OF THE ARTERIES OF THE BRAIN AND SPINAL CORD Part 1 By LESLIE G. KILOH, M.D., M.R.C.P., D.P.M. First Assistant in the Joint Department of Psychological Medicine, Royal Victoria Infirmary and University of Durham Introduction general circulatory efficiency at the time of the Little interest is shown at present in the syn- catastrophe is of the greatest importance. An dromes of the arteries of the brain and spinal cord, occlusion, producing little effect in the presence of and this perhaps is related to the tendency to a normal blood pressure, may cause widespread minimize the importance of cerebral localization. pathological changes if hypotension co-exists. Yet these syndromes are far from being fully A marked discrepancy has been noted between elucidated and understood. It must be admitted the effects of ligation and of spontaneous throm- that in many cases precise localization is often of bosis of an artery. The former seldom produces little more than academic interest and ill effects whilst the latter frequently determines provides Protected by copyright. nothing but a measure of personal satisfaction to infarction. This is probably due to the tendency the physician. But it is worth recalling that the of a spontaneous thrombus to extend along the detailed study of the distribution of the bronchi affected vessel, sealing its branches and blocking and of the pathological anatomy of congenital its collateral circulation, and to the fact that the abnormalities of the heart, was similarly neglected arterial disease is so often generalized. -

Number Reading in Pure Alexiaâ
Neuropsychologia 49 (2011) 2283–2298 Contents lists available at ScienceDirect Neuropsychologia jo urnal homepage: www.elsevier.com/locate/neuropsychologia Reviews and perspectives Number reading in pure alexia—A review a,∗ b Randi Starrfelt , Marlene Behrmann a Center for Visual Cognition, Department of Psychology, Copenhagen University, O. Farimagsgade 2A, DK-1353 Copenhagen K, Denmark b Department of Psychology, Carnegie Mellon University, Pittsburgh, PA, USA a r t i c l e i n f o a b s t r a c t Article history: It is commonly assumed that number reading can be intact in patients with pure alexia, and that this Received 25 October 2010 dissociation between letter/word recognition and number reading strongly constrains theories of visual Received in revised form 31 March 2011 word processing. A truly selective deficit in letter/word processing would strongly support the hypothesis Accepted 22 April 2011 that there is a specialized system or area dedicated to the processing of written words. To date, however, Available online 4 May 2011 there has not been a systematic review of studies investigating number reading in pure alexia and so the status of this assumed dissociation is unclear. We review the literature on pure alexia from 1892 to Keywords: 2010, and find no well-documented classical dissociation between intact number reading and impaired Pure alexia letter identification in a patient with pure alexia. A few studies report strong dissociations, with number Alexia without agraphia reading less impaired than letter reading, but when we apply rigorous statistical criteria to evaluate Letter-by-letter reading Visual recognition these dissociations, the difference in performance across domains is not statistically significant. -

High-Yield Neuroanatomy
LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page i Aptara Inc. High-Yield TM Neuroanatomy FOURTH EDITION LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page ii Aptara Inc. LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page iii Aptara Inc. High-Yield TM Neuroanatomy FOURTH EDITION James D. Fix, PhD Professor Emeritus of Anatomy Marshall University School of Medicine Huntington, West Virginia With Contributions by Jennifer K. Brueckner, PhD Associate Professor Assistant Dean for Student Affairs Department of Anatomy and Neurobiology University of Kentucky College of Medicine Lexington, Kentucky LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page iv Aptara Inc. Acquisitions Editor: Crystal Taylor Managing Editor: Kelley Squazzo Marketing Manager: Emilie Moyer Designer: Terry Mallon Compositor: Aptara Fourth Edition Copyright © 2009, 2005, 2000, 1995 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in the United States of America. All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appearing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at http://www.lww.com (products and services). -

Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000309 ISSN: 2329-6895
olog eur ica N l D f i o s l o a r n d r e u r s o J Lee, et al., J Neurol Disord 2016, 4:7 Journal of Neurological Disorders DOI: 10.4172/2329-6895.1000309 ISSN: 2329-6895 Case Report Open Access Two Cases with Cerebral Infarction in the Left Middle Frontal Lobe Presented as Gerstmann's Syndrome Eun-Ju Lee, Hye-Young Shin, Young Noh, Ki-Hyung Park, Hyeon-Mi Park, Yeong-Bae Lee, Dong-Jin Shin, Young Hee Sung and Dong Hoon Shin* Department of Neurology, Gil Hospital, Gachon University Gil Medical Center, Incheon, South Korea *Corresponding author: Dong Hoon Shin, Department of Neurology, Gil Hospital, Gachon University Gil Medical Center, South Korea, Tel: +82-32-460-3346; Fax: +83-32-460-3344; E-mail: [email protected] Rec date: Oct 08, 2016, Acc date: Oct 18, 2016, Pub date: Oct 22, 2016 Copyright: © 2016 Lee, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Gerstmann's syndrome is a neuropsychological disorder characterized by four symptoms, namely, acalculia, finger agnosia, left-right disorientation, and agraphia suggesting the presence of a lesion in the inferior parietal lobule of the dominant hemisphere, especially at the angular gyrus. Several descriptions of Gerstmann's syndrome have been reported in associated with a lesion to the left frontal lobe, but none of these reports fulfilled the full tetrad of diagnostic criteria.