Chapter 1: Background and Objectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

Top 5 Take-Over Weeds Often Overlooked
14 HOME & GARDEN, DAILY COURIER, Grants Pass, Oregon • FRIDAY, APRIL 12, 2019 Top 5 take-over weeds often overlooked If you read through the list of noxious insects was initially prized. The Oregon where it is poisonous to livestock. weeds published by the Oregon Depart- Department of Agriculture named Buddle- St. Johnswort, Hypericum perforatum, ment of Agriculture, you may be surprised ja davidii as a noxious weed in 2010. is on the Oregon Department of Agricul- to see some old plant friends on the list. Butterfly Bush is a prolific seeder and ture noxious weed list. Tamer cousin St You may have been fighting to keep can easily spread by wind, water, or ani- John’s Wort, Hypericum kouytchense, is them under control mal contact. reportedly an invasive weed in Hawaii, but in your yard, but Reliably sterile varieties available that those who have a garden space where its Story by never considered are somewhat smaller and less showy aggressive nature can be successfully con- them weeds. Are than their invasive cousin are available. trolled may find it to be a useful ground Carolyn McCord they? More defini- And other attractive Buddleja cultivars cover that will grow when other plants tion of “weed” are available. Oregon State University struggle. Keeping control requires vigi- might help. provides information on landscape alter- lance. Avoid planting adjacent to woods, If you search online you will find that natives at pastures or untended land. Wikipedia says a weed is “a plant consid- landscapeplants.oregonstate.edu. • Scotch Broom, Cytisus scoparius, is a ered undesirable in a particular situation; • English Ivy Hedera helix is the lush common plant in our area but one to a plant in the wrong place.” green ivy ground cover and climber seen in avoid. -

Survey of Roadside Alien Plants in Hawai`I Volcanoes National Park and Adjacent Residential Areas 2001–2005
Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANts IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 Linda W. Pratt1 Keali`i F. Bio2 James D. Jacobi1 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kilauea Field Station, P.O. Box 44, Hawaii National Park, HI 96718 2 Hawai‘i Cooperative Studies Unit, University of Hawai‘i at Hilo, P.O. Box 44, Hawai‘i National Park, HI 96718 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This product was prepared under Cooperative Agreement CA03WRAG0036 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANTS IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 1 2 1 LINDA W. PRATT , KEALI`I F. BIO , AND JAMES D. JACOBI 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kīlauea Field Station, P.O. Box 44, Hawai`i Volcanoes National Park, HI 96718 2 Hawaii Cooperative Studies Unit, University of Hawai`i at Hilo, Hilo, HI 96720 Hawai`i Cooperative Studies Unit University of Hawai`i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices ( http://pubs.usgs.gov/circ/1367/ ). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. -

Retail Location List
RETAIL NURSERY AVAILABILITY LIST & Plant Locator: Updated 9/24/21 Here at Annie’s we grow thousands of different plant species right on site. We grow from seeds, cuttings and plugs and offer all plants in 4” containers. Plants available in our retail nursery will differ than those we offer online, and we generally have many more varieties available at retail. If a plant is listed here and not available online, it is not ready to ship or in some cases we are unable to ship certain plants. PLEASE NOTE: This list is updated every Friday and is the best source of information about what is in stock. However, some plants are only grown in small quantities and thus may sell quickly and not be available on the day you arrive at our nursery. The location codes indicate the section and row where the plant is located in the nursery. If you have questions or can’t find a plant, please don’t hesitate to ask one of our nursery staff. We are happy to help you find it. 9/24/2021 ww S ITEM NAME LOCATION Abutilon 'David's Red' 25-L Abutilon striatum "Redvein Indian Mallow" 21-E Abutilon 'Talini's Pink' 21-D Abutilon 'Victor Reiter' 24-H Acacia cognata 'Cousin Itt' 28-D Achillea millefolium 'Little Moonshine' 35-B ww S Aeonium arboreum 'Zwartkop' 3-E ww S Aeonium decorum 'Sunburst' 11-E ww S Aeonium 'Jack Catlin' 12-E ww S Aeonium nobile 12-E Agapanthus 'Elaine' 30-C Agapetes serpens 24-G ww S Agastache aurantiaca 'Coronado' 16-A ww S Agastache 'Black Adder' 16-A Agastache 'Blue Boa' 16-A ww S Agastache mexicana 'Sangria' 16-A Agastache rugosa 'Heronswood Mist' 14-A ww S Agave attenuata 'Ray of Light' 8-E ww S Agave bracteosa 3-E ww S Agave ovatifolia 'Vanzie' 7-E ww S Agave parryi var. -
Pollination, Mating System, Phenology and Characterisation of Medinilla Multiflora Merr
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/329683248 Pollination, Mating System, Phenology and Characterisation of Medinilla multiflora Merr. (Melastomataceae) on Mt. Makiling, Philippines Article · July 2018 DOI: 10.24823/Sibbaldia.2018.251 CITATION READS 1 582 1 author: J. Peter Quakenbush University of the Philippines Los Baños 3 PUBLICATIONS 9 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Medinilla View project All content following this page was uploaded by J. Peter Quakenbush on 15 December 2018. The user has requested enhancement of the downloaded file. SIBBALDIA: 121 The Journal of Botanic Garden Horticulture, No. 16 POLLINATION, MATING SYSTEM, PHENOLOGY AND CHARACTERISATION OF MEDINILLA MULTIFLORA MERR. (MELASTOMATACEAE) ON MT MAKILING, PHILIPPINES J. Peter Quakenbush1 ABSTRACT An investigation into the reproductive biology of Medinilla multiflora Merr. (Melastomataceae) from Mt Makiling, Luzon, is presented. This includes a morphological and distributional exami- nation of the population on the mountain, the documentation of reproductive phenological patterns, a study of the mating system and observations of biotic interactions. Measurements were made of trait variability, reproductive phenology was characterised from field and herbarium observations, stigmatic receptivity was tested by counting pollen germination, insect exclusion and hand-pollination experiments helped determine the mating system and field observations recorded the identity and behaviour of floral visitors. Significant reproductive morphological differences were found between described populations. This identified a need for the recognition of this diversity and further delimitation of the Medinilla multiflora species complex. Although Medinilla multiflora produced flowers and fruit year-round, the population also exhibited cycles of increased reproduction most likely initiated by seasonal low temperatures. -

Pertelaan Morfologi Medinilla Spp. Di Kebun Raya “Eka Karya” Bali Dalam Rangka Pengembangan Tanaman Hias
PERTELAAN MORFOLOGI MEDINILLA SPP. DI KEBUN RAYA “EKA KARYA” BALI DALAM RANGKA PENGEMBANGAN TANAMAN HIAS MORPHOLOGICAL DESCRIPTION OF MEDINILLA SPP. IN BALI BOTANIC GARDEN IN ORDER TO DEVELOP AS ORNAMENTAL PLANT I Nyoman Peneng dan Wawan Sujarwo UPT Balai Konservasi Tumbuhan Kebun Raya “Eka Karya” Bali–LIPI Candikuning, Baturiti, Tabanan 82191. Telp. (0368) 2033170, Fax. (0368) 2033171 e-mail: [email protected]; [email protected] ABSTRACT The morphological description of 13 species of Medinilla spp. (Melastomataceae) in Bali Botanic Garden has been conducted based on morphological characters, so that it could be evidence to be justifi ed as ornamental plant. The study was conducted on plant collections of Bali Botanic Garden and the species of Medinilla which is growing wild inside the garden. Morphological data and agronomic potential of each species were showed in order to support its development as ornamental plant. Based on the results of observation of morphological characterization, several species of Medinilla was suitable to be developed as ornamental plant in pots or planted directly in the fi eld. Further explanation of each Medinilla as ornamental plant will be discussed in this paper. Keywords: morphology, Medinilla spp., Bali Botanic Garden, ornamental plant ABSTRAK Pertelaan morfologi dari 13 jenis Medinilla spp. (Melastomataceae) di Kebun Raya Bali telah dilakukan dengan didasarkan pada karakter sifat morfologi, sehingga hal tersebut dapat menjadi bukti untuk menjustifi kasi sebagai tanaman hias. Penelitian dilakukan pada koleksi Medinilla Kebun Raya Bali dan juga spesies Medinilla yang tumbuh liar (pra koleksi) di dalam kebun. Data morfologi dan potensi agronomi dari setiap spesies Medinilla ditunjukkan untuk mendukung pengembangannya sebagai tanaman hias. -

Tutsan Local Priority Weed
Tutsan Local Priority Weed Under the NSW Biosecurity Act 2015, all landholders have a “General Biosecurity Duty” to manage any “Biosecurity Risk” posed or likely to be posed by Priority Weeds. These weeds can impact on human health, the economy, the liveability of our City and the environment. Impacts can include allergies and other health issues, costs of control, loss of tourism value, degradati on of natural landscapes, parks and recreati on faciliti es, reducti on of useful agricultural land and loss of primary producti on, loss of biodiversity and water quality. MANDATORY OUTCOME The plant should be fully and conti nuously suppressed and destroyed. Scienti fi c name Hypericum androsaemum and Hypericum kouytchense (syn.H. x moserianum) (Family: Clusiaceae) Descripti on Habit: A semi-evergreen shrub to 1.5 m. Stems are reddish and soft wooded, someti mes arching. Leaves: Leaves are stalk-less, green on the upper surface, paler greyish-green below, some turning red during autumn. When crushed, leaves have a slight curry-like aroma Flowers: Flowering in summer. Yellow fl owers are in clusters on the ti ps of branches. Fruit: Fruit contains many oval shaped brown seeds. Hypericum androsaemum has a fl eshy, berry- like fruit that turns red in autumn. The fruit of Hypericum kouytchense is a dry capsule. Photo source: J. Hill Photo source: P. Chrismas Blue Mountains City Council - Bushland Operati ons Team |htt p://www.bmcc.nsw.gov.au/weeds| Phone: 4780 5000 Fact Sheet Fact WEEDS ARE EVERYBODY’S RESPONSIBILITY zb3406loc-tutsan_bmc.indd 1 3/09/2018 4:00 PM Tutsan Local Priority Weed Dispersal Tutsan seeds do not have any special adaptati on for dispersal but are produced in large numbers. -

Impatiens Sodenii Engl. Y Warb (Balsaminaceae), Una Especie Ornamental De Altura En El Salvador
BIOMA, Nº 49, Año 5, agosto 2019 Impatiens sodenii Engl. y Warb (Balsaminaceae), una especie ornamental de altura en El Salvador Resumen Se describe Impatiens sodenii, una hierba sufrutiscente introducida en el país y cultivada en Cerro El Pital, por el momento representa el único registro para El Salvador. Considerada relevante debido a que la familia Balsaminaceae, raramente presenta especies de tamaño arbustivo. Galán, P. Palabras clave: Impatiens, Ornamental, Distribución, El Salvador, Usos, Herbario LAGU, Jardín Botánico La Laguna, Balsaminaceae. Urbanización Industrial Plan de La Laguna, Antiguo Cuscatlán, La Libertad, El Salvador. Correo electrónico: [email protected] Abstract It is described Impatiens sodenii, an introduced suffrutiscent species for the country and cultivated in Cerro El Pital, for the moment represents the only record for El Salvador. Considered relevant because the family Balsaminaceae, rarely presents species of shrub size. Keywords: Impatiens, Ornamental, Distribucion, El Salvador, Usos, Balsaminaceae. 7 ISSN 2307-0560 BIOMA, Nº 49, Año 5, agosto 2019 Introducción Clasificación taxonómica Descripción La familia Balsaminaceae, posee tallos muy suculentos, Reino: Plantae Es una planta herbácea o arbustiva perenne, que crece flores muy vistosas, con colores que van en tonos División: Magnoliophyta hasta 3 metros de altura; sus tallos son suculentos y rosado claro a morado, y de rojo a naranja, la flor glabros, con el tiempo se vuelven leñosos en la base; presenta un espolón en la parte abaxial, lo cual es una Clase: Equisetopsida las hojas son sésiles o cortamente pecioladas, glabras característica de la familia Balsaminaceae, además su Orden: Ericales y los márgenes serrulados; presenta inflorescencias fruto es una cápsula loculicida que al madurar se abre Familia: Balsaminaceae axilares con una o dos flores, las cuales son grandes lanzando las semillas con fuerza, logrando mayor y vistosas, con colores que van desde rosado claro Género: Impatiens éxito de dispersión. -
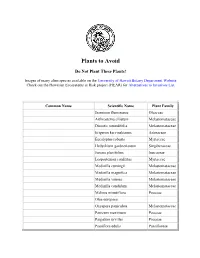
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

Task Force on Landscape Heritage and Plant Diversity Has Determined Initial Designations
THE UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL TASK FORCE ON LANDSCAPE HERITAGE & PLANT DIVERSITY nd 2 EDITION APPROVED BY THE CHANCELLORS BUILDINGS AND GROUNDS COMMITTEE February, 2005 This report is the product of a more than one-year-long effort from concerned members of the University of North Carolina community to ensure that the culturally, historically, and ecologically significant trees and landscaped spaces of the Chapel Hill campus are preserved and maintained in a manner befitting their beauty and grandeur. At the time of this writing, Carolina is in the middle of the most significant building and renovation period in its history. Such a program poses many significant challenges to the survival and well-being of our cherished trees and landscapes. This report attempts to identify, promote awareness, and provide guidelines for both the protection and enhancement of the grounds of the University of North Carolina at Chapel Hill. Furthermore, this report is intended to work within the framework of two earlier documents that help guide development of the campus: the 2002 UNC Master Plan and the 1997 Report of the Chancellor’s Task Force on Intellectual Climate at UNC. We hope that members of the university community as well as outside consultants and contractors will find this information both useful and pertinent. The Taskforce on Landscape Heritage and Plant Diversity 1 This report is the product of a more than one-year-long effort from concerned members of the University of North Carolina community to ensure that the culturally, historically, and ecologically significant trees and landscaped spaces of the Chapel Hill campus are preserved and maintained in a manner befitting their beauty and grandeur. -

Contra Costa Master Gardeners University of California Cooperative Extension
CONTRA COSTA MASTER GARDENERS UNIVERSITY OF CALIFORNIA COOPERATIVE EXTENSION 2014 Sustainability Fair — Ornamental Plants Available for Sale Common Latin Name Name Description Cultivation (1) For most plants, water requirements have been obtained from WUCOLS IV, Water Use Classification of Landscape Species, available on the Web at http://ucanr.edu/sites/WUCOLS/. Abelia ⨉ grandiflora Glossy Abelia A striking variegated plant with a creamy • USDA Zones 6a – 9b ‘Confetti’ margin that turns pinkish red in autumn; • Perennial fragrant white flowers are produced for • Full sun to part shade many months, it is a sport of 'Sherwood' but • Moderate water (1) is even dwarfer, growing 18-24” high with a greater spread, it is ideal for massing and ground cover applications. Abelia ⨉ grandiflora Glossy Abelia Evergreen, compact and dense low • USDA Zones 6 - 10 ‘Kaleidoscope’ mounding shrub to 24-30 inches tall by 36 to • Perennial 42 inches wide with bright red stems holding • Full sun to part shade strongly variegated leaves. Blooms late • Moderate water (1) summer with white tubular flowers persisting into fall. Excellent for containers, as a low accent, and for mass planting. Foliage does not scorch or bleach in full sun. Abutilon cv. - red Flowering This is a red-flowering maple. Makes an • USDA Zones 7b to 10b Maple upright clump to 6' tall x 3' wide flowering • Perennial from late summer and continuing into late • Sun to part shade fall. These bell-shaped flowers are a • Moderate water (1) hummingbird favorite. Agastache ‘Ava’ Ava Mexican Renowned for its tall spikes of deep rose- • USDA Zones 6a to 9b Hyssop pink flowers held by raspberry-red calyxes. -

Melastomataceae) of Borneo
BLUMEA 35 (1990) 5-70 Revision of Medinilla(Melastomataceae) of Borneo Jacinto+C. Regalado+Jr. Department of Botany and Plant Pathology, Michigan State University, East Lansing, Michigan 48824,U. S. A.* Summary Forty-eight species ofMedinilla are now known from Borneo, 28 of which are describedas new. At least 20 taxa are known only from one to three collections. Eleven species groups have been of the awaits further of recognized and defined.A more thorough understanding genus study Philip- pine and New Guinea materials. A key to the Bornean species, illustrations of 15 species, and eco- logical notes are provided. Two previously described species are recorded for the first time for Bor- neo: Medinilla succulenta (Blume) Blume, and M. pterocaula Blume. One new combinationand five reductions have been made. Medinilla tawaensis Merrill is transferred to Catanthera; M. caudatifolia Schwartz and M. hasseltii Blume var. subsessilis Schwartz are reduced to M. crassifolia (Reinw. ex Blume) Blume; M. dajakorum Schwartz is reduced to M. corallina Cogn.; M. borneensis Blume and M. motleyi Hook. f. ex Triana are conspecific with M. macrophylla Blume. Introduction Medinilla (Melastomataceae) is a genus of epiphytic and terrestrial shrubs and climbersof the Paleotropics. It includes about 400 species (Shaw, 1973) distributed in Africa, Madagascar, India, Ceylon, Burma, Indochina,southern China, Thailand, the Malay Peninsula, and eastward to the islands of the Malay Archipelago, New Guinea, northern Australia, Micronesia, and Melanesia. It is by far the largest of all melastome genera occurring in Malesia, a floristic region made up of the Malay Peninsula and islands of the Malay Archipelago extending to New Guinea.