The Interactive Periodic Table of the Elements Teacher's Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

United States Patent (19) 11 Patent Number: 4,640,713 Harris 45 Date of Patent: Feb
United States Patent (19) 11 Patent Number: 4,640,713 Harris 45 Date of Patent: Feb. 3, 1987 (54) TARNESHREMOVER/METAL POLISH 3,458,300 7/1969 Duvall et al. ........................... 106/3 FORMULATION COMPRISING A METAL 3,502,503 3/1970 Bartlo et al. ............................ 134/2 IODIDE, ANACID, AND WATER 3,518,098 6/1970 Ford et al. .............................. O6/3 3,582,366 6/1971 Brieger et al. 75 Inventor: Robert B. Harris, Racine County, 3,687,855 8/1972 Halpern..... Wis. 3,879,216 4/1975 Austin ..................................... 134/4 3,914, 161 10/1975 Yonezawa et al. a 73 Assignee: S. C. Johnson & Son, Inc., Racine, 3,997,460 12A1976 Sirine ............. Wis. 4,097,590 6/1978 Weisz ..... 4,116,699 9/1978 Rooney...... ...10673 21 Appl. No.: 672,966 4,207,310 6/1980 Langford ... ... 252A106 22) Filed: Nov. 19, 1984 4,444,756 4/984 Schlissier ........................... 252/106 51 Int. Cl. .......................... C09G 1/04; C09G 1/06 Primary Examiner-Theodore Morris (52) U.S. C. ........................................... 106/3; 106/10 57 ABSTRACT 58) Field of Search ............................. 106/3; 252/106 A tarnish remover/metal polish formulation comprising 56 References Cited water, an acid, and metal iodide, such as potassium U.S. PATENT DOCUMENTS iodide, is described. The components of the formulation chemically react with the tarnish or stain on a metal 840,167 1/1907 Springborn et al. .................... 106/6 1,280,939 0/1918 Allen ................ ... 06/3 surface, removing the tarnish or stain while leaving the 1,737,222 11/1929 Dewey, Jr. ... 106/9 metal unaffected. -

Copper Alloys
THE COPPER ADVANTAGE A Guide to Working With Copper and Copper Alloys www.antimicrobialcopper.com CONTENTS I. Introduction ............................. 3 PREFACE Conductivity .....................................4 Strength ..........................................4 The information in this guide includes an overview of the well- Formability ......................................4 known physical, mechanical and chemical properties of copper, Joining ...........................................4 as well as more recent scientific findings that show copper has Corrosion ........................................4 an intrinsic antimicrobial property. Working and finishing Copper is Antimicrobial ....................... 4 techniques, alloy families, coloration and other attributes are addressed, illustrating that copper and its alloys are so Color ..............................................5 adaptable that they can be used in a multitude of applications Copper Alloy Families .......................... 5 in almost every industry, from door handles to electrical circuitry to heat exchangers. II. Physical Properties ..................... 8 Copper’s malleability, machinability and conductivity have Properties ....................................... 8 made it a longtime favorite metal of manufacturers and Electrical & Thermal Conductivity ........... 8 engineers, but it is its antimicrobial property that will extend that popularity into the future. This guide describes that property and illustrates how it can benefit everything from III. Mechanical -

The Care and Preservation of Historical Silver by CLARA DECK, CONSERVATOR REVISIONS by LOUISE BECK, CONSERVATOR
The Care and Preservation of Historical Silver BY CLARA DECK, CONSERVATOR REVISIONS BY LOUISE BECK, CONSERVATOR Introduction Historical silver can be maintained for years of use and enjoyment provided that some basic care and attention is given to their preservation. The conservation staff at The Henry Ford have compiled the information in this fact sheet to help individuals care for their objects and collections. The first step in the care of all collections is to understand and minimize or eliminate conditions that can cause damage. The second step is to follow basic guidelines for care, handling and cleaning. Most people know that silver is a white, lustrous metal. Pure or “fine” silver is called “Sterling” if it is made up of no less than 925 parts silver to 75 parts alloy. Sterling will thus often have ‘.925’ stamped somewhere on it, as an identifier. Silver objects, especially coins and jewelry, contain copper as an alloying metal for added hardness. The copper may corrode to form dark brown or green deposits on the surface of the metal. Silver is usually easy to differentiate from lead or pewter, which are generally dark gray and not very shiny. Silver is often plated (deposited) onto other metallic alloys, almost always with an intermediate layer of copper in between. The earliest plating process, “Sheffield Plate” was developed in England in 1742. By the mid-19th century, the process was largely replaced by electroplating (which used less silver). The base metal in plated artifacts may consist of any of the following metals or alloys: copper, brass, “German silver” or “nickel silver” (50% copper, 30% nickel, 20% zinc), “Brittania metal” (97% tin, 7% antimony, 2% copper), or a “base” silver containing a high percentage of copper. -

H2S Pollution and Its Effect on Corrosion of Electronic Components
Chapter 13 H2S Pollution and Its Effect on Corrosion of Electronic Components Benjamin Valdez Salas, Michael Schorr Wiener, Gustavo Lopez Badilla, Monica Carrillo Beltran, Roumen Zlatev, Margarita Stoycheva, Juan de Dios Ocampo Diaz, Lidia Vargas Osuna and Juan Terrazas Gaynor Additional information is available at the end of the chapter http://dx.doi.org/10.5772/39247 1. Introduction The microelectronic industry applies materials with good electrical and corrosion resistance properties for the manufacturing of the microelectronic devices. Silver and copper are materials with this characteristics used for that purpose. Some of their main applications are as high thermal conductive die attach paste that had silver flakes as conductive filler material, silver plated over copper frames, Sn-Ag and Sn-Cu alloys for solder paste used in Surface Mount Technology (SMT) process, conductive internal copper layers in printed circuit boards, copper wire bonding, and more. Other metals and alloys, widely used for support frames, heat diffusers and case wares in the electronics industry are tin, nickel, aluminum, carbon steel and galvanized steel. Corrosion in microelectronics depends on several variants such as the package type, materials involved, assembly processes, moisture, inorganic and organic contaminants, atmospheric pollutants, temperature, thermal stress and electrical bias.(P. Roberge, 2000, J. Payer, 1990, M.Reid et al., 2007, M. Tullmin and P. Roberge, 1995, M. McNeil and B. Little, 1992, X. Lin and J. Zhang, 2004) The plastic packages are the more widely used because of its size, cost and manufacturability advantages but, compared to other hermetic package systems like ceramics, are non-hermetic due to its polymeric materials that permit the permeation of moisture and corrosive gases, allowing in this manner the appearance of other problems associated with corrosion of the inside components. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

DECORATIVE CHROMIUM PLATING I=O44-5 by Donald L
DECORATIVE CHROMIUM PLATING I=o44-5 by Donald L. Snyder 2$& f Engelhard Corp., Beachwood, OH 13lcctrotlcposition of chromium is thc principii1 tncitns of iiiip:trting thc physicill itntl clicniical propcrtics ol’chromium 10 thc surface of lcss cxpcnsive antl casier to lbrm matcriiils such as steel and plastics. The most desirable properties of chromium as a metal coating are its inherent protective and decorative characteristics. The deposit’s high reflectively is retained in service because of chromium’s excellent tarnish, corrosion, wear and scratch resistance. Chromium is almost exclusively plated over a nickel electrodeposit which can easily be plated over substrates such as plastics, steel, aluminum, copper alloys, and zinc die castings. Nickel is preferred because it protects the substrate from corrosion, helps give chromium a white color, and is protected from surface oxidation by the chromium. Stainless steel is the only substrate that is frequently plated directly with chromium, but a nickel preplate before chromium is also used. Multiple or single layers of nickel and copper can precede the nickel/chromium deposits depending upon the intended use of the part. Decorative chromium deposits typically are plated in the 2 to 20 millionths of an inch range. Thicker deposits tend to be duller and contain visible cracks. The traditional chromium deposit is produced from an electroplating electrolyte contain- ing hexavalent chromium ions and has a pleasing bluish-white decorative appearance. About 1975, a chromium electrolyte containing the less toxic and less hazardous trivalent chromium ion began replacing many decorative hexavalent chromium electroplating installations. De- pending upon the process, trivalent chromium electrolytes can either produce a metallic white deposit almost identical in appearance to the bluish-white hexavalent chromium deposits or a deep looking pewter or stainless steel appearing deposit. -

Team Metal Finishing Inc
Team Metal Finishing Inc. Available Processes: Anodizing Thank you for considering Team Metal Finishing for your Hard Coating processing requirements. Team Metal Finishing has been in Electroless Nickel business since 1988 serving the Aerospace, Automotive, Military, Sulfamate Nickel Electronics, Motion Controls and Medical Industries. Since our Bright and Matte Nickel founding we have developed a stellar reputation within these Silver Plating industries for meeting and exceeding the needs and requirements Gold Plating of our customers. We are a ISO 9001:2015 certified company Tin Plating with Aerospace accreditations. We have more than 60,000 Chromate Conversion Coating square feet of processing area on 7.6 acres located in Eastanollee Immersion Tin Ga. Passivation Electropolishing Please give us a call to see what our Team can do for you. www.teammetalfinishing.com 1-800-380-3371 [email protected] Process List: Anodizing Clear & Colors: Black, Blue, Red, Orange and Gold Hard Coat Clear or Natural, Colors: Black, Blue, Red, Orange and Gold Electroless Nickel Medium and High phosphorus; Black Electroless Nickel Nickel Plating Sulfamate, Bright, Matte, and Satin Silver Plating Matte, Semi Bright and Bright Gold Plating 24K Tin Plating Bright and Immersion Tin Chromate Conversions on Aluminum Color: Clear. Trivalent, RoHs. Passivation and Electropolishing Stainless Steels only Abrasive Blasting Glass bead blasting Baking Hydrogen Embrittlement relief Teflon Bonded used with all our coatings www.teammetalfinishing.com 1-800-380-3371 [email protected] Anodizing: Anodizing is an electrolytic process that forms an aluminum oxide onto the surface of the material. The oxide film that is formed grows from the base metal as an integral part of the material. -
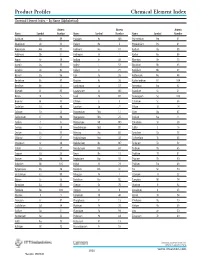
Product Profiles Chemical Element Index
Product Profiles Chemical Element Index Chemical Element Index – By Name (Alphabetical) Atomic Atomic Atomic Name Symbol Number Name Symbol Number Name Symbol Number Actinium Ac 89 Hassium Hs 108 Promethium Pm 61 Aluminum Al 13 Helium He 2 Protactinium Pa 91 Americium Am 95 Holmium Ho 67 Radium Ra 88 Antimony Sb 51 Hydrogen H 1 Radon Rn 86 Argon Ar 18 Indium In 49 Rhenium Re 75 Arsenic As 33 Iodine I 53 Rhodium Rh 45 Astatine At 85 Iridium Ir 77 Rubidium Rb 37 Barium Ba 56 Iron Fe 26 Ruthenium Ru 44 Berkelium Bk 97 Krypton Kr 36 Rutherfordium Rf 104 Beryllium Be 4 Lanthanum La 57 Samarium Sm 62 Bismuth Bi 83 Lawrencium Lr 103 Scandium Sc 21 Boron B 5 Lead Pb 82 Seaborgium Sg 106 Bromine Br 35 Lithium Li 3 Selenium Se 34 Cadmium Cd 48 Lutetium Lu 71 Silicon Si 14 Calcium Ca 20 Magnesium Mg 12 Silver Ag 47 Californium Cf 98 Manganese Mn 25 Sodium Na 11 Carbon C 6 Meitnerium Mt 109 Strontium Sr 38 Cerium Ce 58 Mendelevium Md 101 Sulfur S 16 Cesium Cs 55 Mercury Hg 80 Tantalum Ta 73 Chlorine Cl 17 Molybdenum Mo 42 Technetium Tc 43 Chromium Cr 24 Neilsborium Ns 107 Tellurium Te 52 Cobalt Co 27 Neodymium Nd 60 Terbium Tb 65 Copper Cu 29 Neon Ne 10 Thallium Tl 81 Curium Cm 96 Neptunium Np 93 Thorium Th 90 Dubnium Db 105 Nickel Ni 28 Thulium Tm 69 Dysprosium Dy 66 Niobium Nb 41 Tin Sn 50 Einsteinium Es 99 Nitrogen N 7 Titanium Ti 22 Erbium Er 68 Nobelium No 102 Tungsten W 74 Europium Eu 63 Osmian Os 76 Uranium U 92 Fermium Fm 100 Oxygen O 8 Vanadium V 23 Fluorine F 9 Palladium Pd 46 Xenon Xi 54 Francium Fr 87 Phosphorus P 15 Ytterbium Yb 70 Gadolinium -

The Alkali Metals
Ri Christmas Lectures® 2012: The Modern Alchemist Teaching Resource - Group 1: The Alkali Metals Overview: This resource contains information regarding Group 1 of the Periodic Table - The Alkali Metals. Included is an overview of the elements that make up the group, and their structure, general reactivity, and specific examples of their reactions. The resource is supported by links to useful materials, and video clips from the 2012 Christmas Lectures®. What are the Alkali Metals? Lithium o Lithium is a silver-grey metal; it is the least dense of all metals, with a density of 533 kg m-3. Lithium reacts strongly with moisture, forming corrosive lithium hydroxide; therefore it should be handled carefully. Treatment with lithium is also a common form of anti-depressant therapy. Sodium o Sodium is a silver grey, soft, ductile metal. It is most commonly seen in everyday life as Sodium chloride, otherwise known as table salt. It is also present in street lighting as the common yellow glow of Sodium vapour lamps. Sodium is also extremely important in the body, where it is involved in nerve impulses. Potassium o Potassium is a soft, silver metal; however when exposed to air it tarnishes quickly. Interestingly, Potassium is both essential for the human biological system and has a naturally radioactive isotope. Despite this, the natural radioactivity from Potassium is low (it has a half life of 1.248 x 109 years, see Teaching Resource - Radioactivity on the RSC Learn Chemistry website for more details on half-life). Rubidium o Rubidium is again a soft metal, however, it is pyrophoric and with ignite spontaneously in moist air. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants
UNLV Theses, Dissertations, Professional Papers, and Capstones 5-1-2015 Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants John Dustin Despotopulos University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Despotopulos, John Dustin, "Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2345. http://dx.doi.org/10.34917/7645877 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INVESTIGATION OF FLEROVIUM AND ELEMENT 115 HOMOLOGS WITH MACROCYCLIC EXTRACTANTS By John Dustin Despotopulos Bachelor of Science in Chemistry University of Oregon 2010 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy