Molecular Epidemiology of Plasmodium Species In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pakistan-U.S. Relations
Pakistan-U.S. Relations K. Alan Kronstadt Specialist in South Asian Affairs July 1, 2009 Congressional Research Service 7-5700 www.crs.gov RL33498 CRS Report for Congress Prepared for Members and Committees of Congress Pakistan-U.S. Relations Summary A stable, democratic, prosperous Pakistan actively combating religious militancy is considered vital to U.S. interests. U.S. concerns regarding Pakistan include regional and global terrorism; Afghan stability; democratization and human rights protection; the ongoing Kashmir problem and Pakistan-India tensions; and economic development. A U.S.-Pakistan relationship marked by periods of both cooperation and discord was transformed by the September 2001 terrorist attacks on the United States and the ensuing enlistment of Pakistan as a key ally in U.S.-led counterterrorism efforts. Top U.S. officials praise Pakistan for its ongoing cooperation, although long-held doubts exist about Islamabad’s commitment to some core U.S. interests. Pakistan is identified as a base for terrorist groups and their supporters operating in Kashmir, India, and Afghanistan. Pakistan’s army has conducted unprecedented and, until recently, largely ineffectual counterinsurgency operations in the country’s western tribal areas, where Al Qaeda operatives and pro-Taliban militants are said to enjoy “safe haven.” U.S. officials increasingly are concerned that indigenous religious extremists represent a serious threat to the stability of the Pakistani state. The United States strongly encourages maintenance of a bilateral cease-fire and a continuation of substantive dialogue between Pakistan and neighboring India, which have fought three wars since 1947. A perceived Pakistan-India nuclear arms race has been the focus of U.S. -

Title Changing Gender Relations on Return from Displacement to The
HPG Report/WorkingHPG Working Paper Changing gender relations on return from displacementTitle to the Subtitlenewly merged districts Authorsof Pakistan Simon Levine Date October 2020 About the author Simon Levine is a Senior Research Fellow at the Humanitarian Policy Group (HPG) at ODI. Acknowledgements This work would not have been possible without a dedicated team of researchers who did not simply conduct the interviews: they managed the whole process of fieldwork and shaped the analysis in this paper by combining their deep familiarity with the area with a very sharp analysis of the changes they saw happening. They know who they are, and they know how great is my debt to them. Thanks, too, to Megan Daigle, Kerrie Holloway and Sorcha O’Callaghan for comments on earlier drafts; and to the (anonymous) peer reviewers who generously gave up their time to give an incisive critique that helped this to become a better paper. Katie Forsythe worked her editing magic, as always; and Hannah Bass ensured that the report made it swiftly through production, looking perfect. Thanks also to Catherine Langdon, Sarah Cahoon and Isadora Brizolara for facilitating the project. The core of HPG’s work is its Integrated Programme (IP), a two-year body of research spanning a range of issues, countries and emergencies, allowing it to examine critical issues facing humanitarian policy and practice and influence key debates in the sector. This paper is part of HPG’s 2019–2021 IP, ‘Inclusivity and invisibility in humanitarian action’. The author would like to thank HPG’s IP donors, whose funding enables this research agenda. -

Pakistan Security Situation
EASO Pakistan Security Situation Country of Origin Information Report October 2020 More information on the European Union is available on the Internet (http://europa.eu). EN PDF/Volume_01 ISBN: 978-92-9485-683-8 doi: 10.2847/737033 BZ-02-20-905-EN-N © European Asylum Support Office, 2020 Cover photo: © PSSP Lahore, Pakistan 2013 url CC BY 2.0 Reproduction is authorised provided the source is acknowledged. For any use or reproduction of photos or other material that is not under the EASO copyright, permission must be sought directly from the copyright holders. Country of origin information report | Pakistan: Security Situation Acknowledgements EASO acknowledges as the drafter of this report: Belgium, Centre for Documentation and Research (Cedoca) in the Office of the Commissioner General for Refugees and Stateless Person The following departments and organisations have reviewed the report: Austria, Federal Office for Immigration and Asylum, Country of Origin Information Department Poland, Country of Origin Information Unit, Department for Refugee Procedures, Office for Foreigners ACCORD – Austrian Centre for Country of Origin and Asylum Research and Documentation It must be noted that the review carried out by the mentioned departments, experts or organisations contributes to the overall quality of the report, but does not necessarily imply their formal endorsement of the final report, which is the full responsibility of EASO. 3 Country of origin information report | Pakistan: Security Situation Contents Acknowledgements ................................................................................................................................ -

Marble Industry Role in the Socio Economic Development of Marble Industrial Owners of District Mohmand Federal Administered Tribal Area-Pakistan
Industrial Engineering Letters www.iiste.org ISSN 2224-6096 (Paper) ISSN 2225-0581 (online) DOI: 10.7176/IEL Vol.9, No.3, 2019 Marble Industry Role in the Socio Economic Development of Marble Industrial Owners of District Mohmand Federal Administered Tribal Area-Pakistan Sajjad Ahmad Institute of Development Studies, The University of Agriculture Peshawar Dr.Naushad Khan Institute of Development Studies, The University of Agriculture Peshawar Abstract The study was carried out in District Mohmand in August, 2018. The major objective was, to find out marble industry role in the socio-economic development of District Mohmand. The study area consists of 7 tehsils while three tehsils namely Safi, Pandiali, Khwezai Bazai were selected on the basis of more marble industries . The total number of marble industries in these tehsils were 140, Safi 40, Pandiali 48 and Khwazia Bazai 48 while all were selected for the present study. Data were collected though questionnaire while Descriptive statistic and paired T-test were used for data analysis. The mean monthly income of the respondents after marble industry was Rs.97286 and before was Rs.49843 while mean monthly expenditure after was Rs.51714 and before was Rs.39479.Similarly the mean monthly saving after marble industrial owners was found Rs.45500 and before was Rs.10786 and the school children were found more than before. Similarly the private school number was found more than before. The monthly mean expenditure on education after marble industry was found Rs.6151 and the expenditure before was Rs.4361. Subsequently the private hospitals were found more than before. -

Government of Khyber Pakhtunkhwa
GOVERNMENT OF KHYBER PAKHTUNKHWA Public Disclosure Authorized Public Disclosure Authorized Qabail Led Community Support Project (QLCSP) Environmental and Social Management Framework (ESMF) Public Disclosure Authorized December 21, 2019 To be executed By Planning & Development Department (GoKP) Through Public Disclosure Authorized Directorate of Projects under the Merged Areas Secretariat (MAS) EXECUTIVE SUMMARY Introduction The Government of Khyber Pakhtunkhwa (GoKP), through Directorate of Projects Planning & Development Department (DP&DD), intends to implement “Qabail Led Community Support Program (QLCSP”) in Khyber district of merged areas (MA) – the erstwhile Federally Administered Tribal Areas (FATA)1 – and Peshawar and Nowshera districts of KP with the proposed assistance of the World Bank (WB).2 This Environmental and Social Management Framework (ESMF) has been prepared to meet requirements of national legislation of Pakistan and World Bank environmental and social policy requirements to address potential negative impacts from the proposed project. Project Overview Background The Central Asia-South Asia Electricity Transmission and Trade Project (CASA1000) aims to facilitate electricity trade between Central Asia and countries in South Asia by putting in place transmission infrastructure. As part of CASA1000 project, each participating country3 is implementing Community Support Programs (CSPs) to share the benefits associated with the project and to generate support among local communities. Project Area In Pakistan, the CASA1000 transmission line (TL) will pass through approximately 100 kilometer long territory passing through various parts of KP province. The project area accordingly lies in/includes Peshawar and Nowshera districts and Khyber district4 of merged areas (MA). Project Components The Project has four components as briefly described below; and its Project Development Objective (PDO) is “improve access to local infrastructure and strengthen community engagement in the project areas”. -

Special Report No
SPECIAL REPORT NO. 494 | MAY 2021 UNITED STATES INSTITUTE OF PEACE www.usip.org The Evolution and Potential Resurgence of the Tehrik-i-Taliban Pakistan By Amira Jadoon Contents Introduction ...................................3 The Rise and Decline of the TTP, 2007–18 .....................4 Signs of a Resurgent TPP, 2019–Early 2021 ............... 12 Regional Alliances and Rivalries ................................ 15 Conclusion: Keeping the TTP at Bay ............................. 19 A Pakistani soldier surveys what used to be the headquarters of Baitullah Mehsud, the TTP leader who was killed in March 2010. (Photo by Pir Zubair Shah/New York Times) Summary • Established in 2007, the Tehrik-i- attempts to intimidate local pop- regional affiliates of al-Qaeda and Taliban Pakistan (TTP) became ulations, and mergers with prior the Islamic State. one of Pakistan’s deadliest militant splinter groups suggest that the • Thwarting the chances of the TTP’s organizations, notorious for its bru- TTP is attempting to revive itself. revival requires a multidimensional tal attacks against civilians and the • Multiple factors may facilitate this approach that goes beyond kinetic Pakistani state. By 2015, a US drone ambition. These include the Afghan operations and renders the group’s campaign and Pakistani military Taliban’s potential political ascend- message irrelevant. Efforts need to operations had destroyed much of ency in a post–peace agreement prioritize investment in countering the TTP’s organizational coherence Afghanistan, which may enable violent extremism programs, en- and capacity. the TTP to redeploy its resources hancing the rule of law and access • While the TTP’s lethality remains within Pakistan, and the potential to essential public goods, and cre- low, a recent uptick in the number for TTP to deepen its links with ating mechanisms to address legiti- of its attacks, propaganda releases, other militant groups such as the mate grievances peacefully. -

Download 2.26 MB
Initial Environmental Examination Report ________________________________________ Project Number: 47021-002 Loan Number: 3239 PAK: Federally Administered Tribal Areas Water Resources Development Project Initial Environmental Examination Report for Warsak Left Bank Canal, District Mohmand Prepared by Project Management Unit, Government of Khyber Pakhtunkhwa, Pakistan For the Asian Development Bank Date received by ADB: August 2020 NOTES (i) The fiscal year (FY) of the Government of the Islamic Republic of Pakistan and its agencies ends on 30 June. (ii) In this report “$” refer to US dollars. This initial environmental examination report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Project Management Unit PMU FATA Water Resources Development Project FWRDP KP P&D Department FEDERALLY ADMINISTERED TRIBAL AREAS WATER RESOURCES DEVELOPMENT PROJECT INITIAL ENVIRONMENTAL EXAMINATION (IEE) COMMAND AREA DEVELOPMENT OF WARSAK LEFT BANK CANAL (MOHMAND DISTRICT) August, 2020 JOINT VENTURE: FATA WATER RESOURCES DEVELOPMENT PROJECT CONSULTANTS House # 3, Street # 1, Near Board Bazar, Tajabad, Peshawar, Khyber Pakhtunkhwa, Pakistan. Tel: +92 91 5601635 - 6 Fax: +92 91 5840807 E-mail: [email protected] Initial Environmental Examination: FATA Water Resources Development Project CADWLBC subproject TABLE OF CONTENTS S. No Description .................................................................................................... Page No. INTRODUCTION ..................................................................................................................... -

Initial Environmental Examination Report ______
Initial Environmental Examination Report ________________________________________ Project Number: 47021-002 Loan Number: 3239 PAK: Federally Administered Tribal Areas Water Resources Development Project Initial Environmental Examination Report for Command Area Development of Raghagan Dam, District Bajaur Prepared by Project Management Unit, Government of Khyber Pakhtunkhwa, Pakistan For the Asian Development Bank Date received by ADB: October 2019 NOTES (i) The fiscal year (FY) of the Government of the Islamic Republic of Pakistan and its agencies ends on 30 June. (ii) In this report “$” refer to US dollars. This initial environmental examination report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Project Management Unit • PMU FATA Water Resources Development Project FWRDP Merged Areas Secretariat FEDERALLY ADMINISTERED TRIBAL AREAS WATER RESOURCES DEVELOPMENT PROJECT INITIAL ENVIRONMENTAL EXAMINATION (IEE) COMMAND AREA DEVELOPMENT OF RAGHAGAN DAM SUB PROJECT (BAJAUR DISTRICT) 2019 JOINT VENTURE: FATA WATER RESOURCES DEVELOPMENT PROJECT CONSULTANTS House # 3, Street # 1, Near Board Bazar, Tajabad, Peshawar, Khyber Pakhtunkhwa, Pakistan. Tel: +92 91 5601635 - 6 Fax: +92 91 5840807 E-mail: [email protected] Initial Environmental Examination: FATA Water Resources Development Project CARD Sub Project TABLE OF CONTENTS S. No. Description Page No. -

Initial Environmental Examination Report ______
Initial Environmental Examination Report ________________________________________ Project Number: 47021-002 Loan Number: 3239 PAK: Federally Administered Tribal Areas Water Resources Development Project Initial Environmental Examination Report for Ganjyano Danish Kool Lift Irrigation District Mohmand Prepared by Project Management Unit, Government of Khyber Pakhtunkhwa, Pakistan For the Asian Development Bank Date received by ADB: July 2019 NOTES (i) The fiscal year (FY) of the Government of the Islamic Republic of Pakistan and its agencies ends on 30 June. (ii) In this report “$” refer to US dollars. This initial environmental examination report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Project Management Unit PMU FATA Water Resources Development Project FWRDP Merged Area Secretariat FEDERALLY ADMINISTERED TRIBAL AREAS WATER RESOURCES DEVELOPMENT PROJECT INITIAL ENVIRONMENTAL EXAMINATION (IEE) GANJYANO DANISH KOOL LIFT IRRIGATION (MOHMAND TRIBAL DISTRICT) July 2019 JOINT VENTURE: FATA WATER RESOURCES DEVELOPMENT PROJECT CONSULTANTS House # 3, Street # 1, Near Board Bazar, Tajabad, Peshawar, Khyber Pakhtunkhwa, Pakistan. Tel: +92 91 5601635 - 6 Fax: +92 91 5840807 E-mail: [email protected] Initial Environmental Examination: FATA Water Resources Development Project Ganjyano Danish Kool subproject TABLE OF CONTENTS S. No. Description Page No. INTRODUCTION......................................................................................................... -
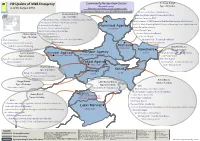
4W Map of NWA Core Cluster V3
4W Update of NWA Emergency Community Restoration Cluster Peshawar District emetic areas Type of Activities as of 05 August 2014 Governance Non-farm Livelihood - CCG Community Infrastructures DRR Environment - Drain Construction / Maintenance Mohmand District TAJIKISTAN - Drinking Water Supply Scheme Installation CHINA Type of Activities Area of Detail A Jammu - Female Home base CFW and Kashmir - Establishing of 20 community based education centers AFGHANISTAN Islamabad - Establishment of IDP Grievance RedResal Machenism (Call Centres) Lahore - Provision of Primary Health Care Services Quetta - Cash for Work/Cleaning/Rehabilitation of drainage lines/ debris removal - Construction of Latrines IRAN Indus Mohmand Agency - Hand Pump Installation INDIA - Provision of micro enterprise grants HIN, IRP, WFP -Agro-Forestry-Livestock activities - Hand Pump Repair Khyber Agency - Vocational training - Pressure Pumps Installation Type of Activities - Poultry distribution - Tube Well Repair - Shelter assistance - Social mobilization and capacity building - Washing Pads - Construction/Repair -Agro-Forestry-Livestock activities -CPI activiities - Waste Management - Skill Development tranining Peshawar Nowshera District ACTED, UNDP - Disaster Risk Reduction Nowshera Type of Activities Kurram Agency Khyber Agency ACTED - Drain Construction / Maintenance Kurram Agency UN-Habitat, FDM, UNESCO,WFP Type of Activities UN-Habitat, FDM, WFP - Hand Pump Repair -Agro-Forestry-Livestock activities - Tube Well Repair - WASH assistance Orakzai Agency - Washing Pads -

ADP 2021-22 Planning and Development Department, Govt of Khyber Pakhtunkhwa Page 1 of 446 NEW PROGRAMME
ONGOING PROGRAMME SECTOR : Agriculture SUB-SECTOR : Agriculture Extension 1.KP (Rs. In Million) Allocation for 2021-22 Code, Name of the Scheme, Cost TF ADP (Status) with forum and Exp. upto Beyond S.#. Local June 21 2021-22 date of last approval Local Foreign Foreign Cap. Rev. Total 1 170071 - Improvement of Govt Seed 288.052 0.000 230.220 23.615 34.217 57.832 0.000 0.000 Production Units in Khyber Pakhtunkhwa. (A) /PDWP /30-11-2017 2 180406 - Strengthening & Improvement of 60.000 0.000 41.457 8.306 10.237 18.543 0.000 0.000 Existing Govt Fruit Nursery Farms (A) /DDWP /01-01-2019 3 180407 - Provision of Offices for newly 172.866 0.000 80.000 25.000 5.296 30.296 0.000 62.570 created Directorates and repair of ATI building damaged through terrorist attack. (A) /PDWP /28-05-2021 4 190097 - Wheat Productivity Enhancement 929.299 0.000 378.000 0.000 108.000 108.000 0.000 443.299 Project in Khyber Pakhtunkhwa (Provincial Share-PM's Agriculture Emergency Program). (A) /ECNEC /29-08-2019 5 190099 - Productivity Enhancement of 173.270 0.000 98.000 0.000 36.000 36.000 0.000 39.270 Rice in the Potential Areas of Khyber Pakhtunkhwa (Provincial Share-PM's Agriculture Emergency Program). (A) /ECNEC /29-08-2019 6 190100 - National Oil Seed Crops 305.228 0.000 113.000 0.000 52.075 52.075 0.000 140.153 Enhancement Programme in Khyber Pakhtunkhwa (Provincial Share-PM's Agriculture Emergency Program). -

Un Khyber Pakhtunkhwa Merged Districts Support Programme Qualitative Research Finding
UN KHYBER PAKHTUNKHWA MERGED DISTRICTS SUPPORT PROGRAMME QUALITATIVE RESEARCH FINDINGS LOCAL GRIEVANCES AND COMPLAINT REGISTRATION MECHANISMS May – June 2019 Verso ConsultIng Pvt. Ltd 1 General FindIngs The UNRCO team has undertaken a research exercise to inform the back-end structure of the programme’s Grievance Redress Mechanism. The qualitative enquiry has been conducted in a selection of 15 tehsils/sub-divisions across 5 NMDs (Khyber, Kurram, Orakzai, North Waziristan, and South Waziristan) with three tehsils selected per district. Through 30 focus group discussions (15 male and 15 female) and 30 key informant interviews the research team has investigated the following: a) status of mobility for different target groups; b) patterns of vulnerability and exclusion; c) access to social and economic services; d) various types of grievances; e) channels of registering complaints; and f) perceptions of the forthcoming transition in rule of law. The research found that mobility is generally problematic for a majority of the population due to lack of transport facilities and fuel service points, however mobility for women is disproportionately restricted across 4 of 5 districts (Khyber being the exception). Additionally in areas with active sectarian conflict (Orakzai and Kurram) mobility is severely restricted for religious minorities and women. While most respondents reported monthly household income under PKR 15,000/- and high poverty remains an excluding factor, exclusion from social and public participation is also a function of gender, disability, and age. Similarly, in access to services, schools, hospitals, rural and remote populations are disproportionately excluded. Local health centers/ BHUs are mostly unreliable in terms of service capacity – those who can afford the travel expense rely on health centers in the district headquarters or travel to big cities.