Association Between a G-Protein Β3 Subunit Gene Polymorphism and The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Data Sheet
Product Data Sheet ExProfileTM Human AMPK Signaling Related Gene qPCR Array For focused group profiling of human AMPK signaling genes expression Cat. No. QG004-A (4 x 96-well plate, Format A) Cat. No. QG004-B (4 x 96-well plate, Format B) Cat. No. QG004-C (4 x 96-well plate, Format C) Cat. No. QG004-D (4 x 96-well plate, Format D) Cat. No. QG004-E (4 x 96-well plate, Format E) Plates available individually or as a set of 6. Each set contains 336 unique gene primer pairs deposited in one 96-well plate. Introduction The ExProfile human AMPK signaling related gene qPCR array profiles the expression of 336 human genes related to AMPK-mediated signal transduction. These genes are carefully chosen for their close pathway correlation based on a thorough literature search of peer-reviewed publications, mainly including genes that encode AMP-activated protein kinase complex,its regulators and targets involved in many important biological processes, such as glucose uptake, β-oxidation of fatty acids and modulation of insulin secretion. This array allows researchers to study the pathway-related genes to gain understanding of their roles in the different biological processes. QG004 plate 01: 84 unique gene PCR primer pairs QG004 plate 02: 84 unique gene PCR primer pairs QG004 plate 03: 84 unique gene PCR primer pairs QG004 plate 04: 84 unique gene PCR primer pairs Shipping and storage condition Shipped at room temperate Stable for at least 6 months when stored at -20°C Array format GeneCopoeia provides five qPCR array formats (A, B, C, D, and E) suitable for use with the following real- time cyclers. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Novel Driver Strength Index Highlights Important Cancer Genes in TCGA Pancanatlas Patients
medRxiv preprint doi: https://doi.org/10.1101/2021.08.01.21261447; this version posted August 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Novel Driver Strength Index highlights important cancer genes in TCGA PanCanAtlas patients Aleksey V. Belikov*, Danila V. Otnyukov, Alexey D. Vyatkin and Sergey V. Leonov Laboratory of Innovative Medicine, School of Biological and Medical Physics, Moscow Institute of Physics and Technology, 141701 Dolgoprudny, Moscow Region, Russia *Corresponding author: [email protected] NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. 1 medRxiv preprint doi: https://doi.org/10.1101/2021.08.01.21261447; this version posted August 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Abstract Elucidating crucial driver genes is paramount for understanding the cancer origins and mechanisms of progression, as well as selecting targets for molecular therapy. Cancer genes are usually ranked by the frequency of mutation, which, however, does not necessarily reflect their driver strength. Here we hypothesize that driver strength is higher for genes that are preferentially mutated in patients with few driver mutations overall, because these few mutations should be strong enough to initiate cancer. -

Cardiovascular Diseases and G-Protein B3 Subunit Gene (GNB3) in the Era of Genomewide Scans
Journal of Human Hypertension (2003) 17, 379–380 & 2003 Nature Publishing Group All rights reserved 0950-9240/03 $25.00 www.nature.com/jhh COMMENTARY Cardiovascular diseases and G-protein b3 subunit gene (GNB3) in the era of genomewide scans M Tomaszewski1,2, FJ Charchar1, S Padmanabhan1, E Zukowska-Szczechowska2, W Grzeszczak2 and AF Dominiczak1 1BHF Glasgow Cardiovascular Research Centre, Division of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; 2Department of Internal Medicine, Diabetology and Nephrology, Medical University of Silesia, Zabrze, Poland Journal of Human Hypertension (2003) 17, 379–380. doi:10.1038/sj.jhh.1001559 The last 4 years were the era of genomewide scans The ability of angiotensin-converting enzyme (ACE) in cardiovascular genetics. Since 1999, after pub- inhibitors to induce the regression of hypertensive lication of the first comprehensive genome linkage LVH and prevent ventricular remodelling after analysis of systolic blood pressure in humans,1 more myocardial infarction provides a clinical correlate than 20 genomewide searches for hypertension to these observations. Thus, insertion/deletion (I/D) genes have been reported. The number of detected polymorphism of the ACE gene has been tested as a cardiovascular quantitative trait loci (QTLs) has potential risk factor of hypertensive LVH in numer- increased rapidly, forming a new dense QTL-map ous studies, and the proportion of negative and within the human genome. At present, a QTL for positive results appears to be roughly equal.2 blood pressure or hypertension exists almost on Studies on association between hypertensive LVH every human chromosome. However, most of the and polymorphisms within the genes encoding detected QTLs do not overlap, even within popula- angiotensinogen and angiotensin II receptors have tions of the same ethnic origin. -

Supplemental Digital Content (Sdc) Sdc, Materials
SUPPLEMENTAL DIGITAL CONTENT (SDC) SDC, MATERIALS AND METHODS Animals This study used 9-12 week old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). This study conformed to the National Institutes of Health guidelines and was conducted under animal protocols approved by the University of Virginia’s Institutional Animal Care and Use Committee. Murine DCD Lung Procedure Mice were anesthetized by isoflurane inhalation and euthanized by cervical dislocation followed by a 60-minute period of “no-touch” warm ischemia. Mice then underwent extended median sternotomy and midline cervical exposure followed by intubation for the initiation of mechanical ventilation at 120 strokes/minute with room air. The left atrium was vented via an atriotomy followed by infusion of the lungs with 3 mL 4°C Perfadex® solution (Vitrolife Inc., Denver, CO) supplemented with THAM Solution (Vitrolife, Kungsbacka, Sweden), estimating weight-based volume recommendations for pulmonary artery perfusion (140mL/kg) (1). The chest was then packed with ice and the trachea occluded by silk-suture tie at tidal volume (7µL/g body weight) prior to cold static preservation (CSP) for 60 minutes at 4°C. Mice were then randomized into three experimental groups: 1) CSP alone with no EVLP, 2) EVLP with Steen solution and 3) EVLP with Steen solution supplemented with the highly selective A2AR agonist, ATL1223 (30nM, Lewis and Clark Pharmaceuticals, Charlottesville, VA). Mice treated with ATL1223 during EVLP also received ATL1223 treatment (30nM) during the Perfadex flush prior to CSP whereas the EVLP group received vehicle (DMSO) during the flush. CSP lungs, which did not undergo EVLP, underwent immediate functional assessment after re-intubation as described below. -

Mouse Model Implicates GNB3 Duplication in a Childhood Obesity Syndrome
Mouse model implicates GNB3 duplication in a childhood obesity syndrome Ian S. Goldlusta, Karen E. Hermetza, Lisa M. Catalanoa, Richard T. Barfieldb, Rebecca Cozada, Grace Wynna, Alev Cagla Ozdemira, Karen N. Conneelya,b, Jennifer G. Mullea,c, Shikha Dharamrupa, Madhuri R. Hegdea, Katherine H. Kimd, Brad Angled, Alison Colleye, Amy E. Webbf, Erik C. Thorlandg, Jay W. Ellisonh, Jill A. Rosenfeldh, Blake C. Ballifh,1, Lisa G. Shafferh,1, Laurie A. Demmeri,2, Unique Rare Chromosome Disorder Support Groupj,3, and M. Katharine Rudda,4 aDepartment of Human Genetics, Emory University School of Medicine, Atlanta, GA 30322; Departments of bBiostatistics and Bioinformatics and cEpidemiology, Emory University School of Public Health, Atlanta, GA 30322; dDivision of Genetics, Northwestern University Feinberg School of Medicine, Chicago, IL 60614; eDepartment of Clinical Genetics, South Western Sydney Local Health District, Liverpool, NSW 1871, Australia; fAmy E. Webb Pediatrics, Pismo Beach, CA 93449; gDepartment of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN 55905; hSignature Genomic Laboratories, PerkinElmer, Inc., Spokane, WA 99207; iDivision of Genetics and Metabolism, Tufts University School of Medicine, Boston, MA 02111; and jUnique Rare Chromosome Disorder Support Group, Surrey CR3 5GN, United Kingdom Edited* by Stephen T. Warren, Emory University School of Medicine, Atlanta, GA, and approved July 29, 2013 (received for review March 29, 2013) Obesity is a highly heritable condition and a risk factor for other proven insightful in teasing apart the physiological effects of diseases, including type 2 diabetes, cardiovascular disease, hyper- genes involved in obesity (21–24). tension, and cancer. Recently, genomic copy number variation (CNV) Here, we report a syndrome caused by a recurrent chromo- has been implicated in cases of early onset obesity that may be somal translocation associated with obesity as well as ID, seizures, comorbid with intellectual disability. -

Association Study of the G-Protein B3 Subunit C825T Polymorphism with Disease Progression an Overall Survival in Patients with Head and Neck Squamous Cell Carcinoma
3203 Association Study of the G-Protein B3 Subunit C825T Polymorphism with Disease Progression an Overall Survival in Patients with Head and Neck Squamous Cell Carcinoma Goetz F. Lehnerdt,1 Peter Franz,1 Agnes Bankfalvi,2 Sara Grehl,3 Klaus Jahnke,1,6 Stephan Lang,1,6 Kurt W. Schmid,2,6 Winfried Siffert,4,6 and Ulrich H. Frey4,5 1Department of Otorhinolaryngology, 2Institute of Pathology and Neuropathology, 3Department of Radiotherapy at the West German Cancer Center Essen, 4Institute of Pharmacogenetics, 5Department of Anaesthesiology and Intensive Care Medicine, and 6West-German Cancer Center Essen, Essen, Germany Abstract The T-allele of a common C825T single nucleotide significant genotype-dependent relapse-free interval polymorphism (SNP) in the gene GNB3, encoding the (P = 0.036). In multivariate analysis with stage, G3 subunit of heterotrimeric G-proteins, is associated localization, grade, gender, and smoking habits with a truncated form of the G3 protein that imparts a as covariates, GNB3 825T homozygous patients greater signaling capacity than the alternative C-allele displayed a higher risk for relapse than C825 homozy- encoding a nontruncated protein. We analyzed gous patients (TT versus CC, hazard ratio; 95% the C825T-allele status with regard to disease progres- confidence interval, 1.4-4.8; P =0.002).Thesame sion in patients with head and neck squamous cell genotype effect was found for overall survival, TT carcinoma (HNSCC). The prognostic value of the SNP genotypes were at higher risk for death compared was evaluated in an unselected series of 341 patients with CC genotypes (hazard ratio, 2.6; 95% confidence treated with curative intent for HNSCC including all interval, 1.6-4.3; P < 0.001), and 5-year survival tumor stages with different therapeutic regimens. -

Gene Associated with Sudden Cardiac Death Identified by ICD Monitoring 31 August 2015
Gene associated with sudden cardiac death identified by ICD monitoring 31 August 2015 A gene associated with sudden cardiac death in ventricular tachyarrhythmias. The researchers the general population has been identified using genotyped seven single nucleotide polymorphisms implantable cardioverter defibrillator (ICD) (SNPs) in three genes (GNB3, GNAQ and GNAS) monitoring in research presented for the first time coding G-protein subunits.2 G-proteins interact with at ESC Congress today. The research included stimulated adrenoreceptors, angiotensin II patients from the DISCOVERY trial and Oregon- receptors and ion channels in myocardial cells. SUDS and discovered that a polymorphism in the Abnormal G-protein signal transduction has been GNAS gene predicted ventricular tachyarrhythmias suggested as a mechanism contributing to sudden and sudden cardiac death. cardiac death. "This is the first time a gene has been identified In the second part of the study, the genes found to using ICD monitoring and then confirmed to be be associated with cardiac arrhythmias in the associated with sudden cardiac death in the DISCOVERY trial were evaluated in 1 335 patients general population," said principal investigator from Oregon-SUDS (Sudden Unexpected Death Professor Heiner Wieneke, chief physician in the Study), a community-based study analysing causes Department of Cardiology, Contilia Heart and of sudden cardiac death in the Portland, Oregon Vessel Centre, St. Marien-Hospital Mülheim, metropolitan area.3 Germany. "Epidemiological studies have suggested that genetic factors contribute to In the DISCOVERY trial, 297 patients had a sudden cardiac death but only a few genes have ventricular tachyarrhythmia. In univariate analysis, been identified." genotypes of two SNPs in the GNAS gene were significantly predictive of ventricular Sudden cardiac death is one of the leading causes tachyarrhythmias. -

SUPPLEMENTAL MATERIAL Acknowledgments
SUPPLEMENTAL MATERIAL Acknowledgments The members of the CARDIoGRAM consortium are: Heribert Schunkert, Inke R. König, Sekar Kathiresan, Muredach P. Reilly, Themistocles L. Assimes, Hilma Holm, Michael Preuss, Alexandre F. R. Stewart, Maja Barbalic, Christian Gieger, Devin Absher, Zouhair Aherrahrou, Hooman Allayee, David Altshuler, Sonia S. Anand, Karl Andersen, Jeffrey L. Anderson, Diego Ardissino, Stephen G. Ball, Anthony J. Balmforth, Timothy A. Barnes, Diane M. Becker, Lewis C. Becker, Klaus Berger, Joshua C. Bis, S. Matthijs Boekholdt, Eric Boerwinkle, Peter S. Braund, Morris J. Brown, Mary Susan Burnett, Ian Buysschaert, Cardiogenics, John F. Carlquist, Li Chen, Sven Cichon, Veryan Codd, Robert W. Davies, George Dedoussis, Abbas Dehghan, Serkalem Demissie, Joseph M. Devaney, Ron Do, Angela Doering, Sandra Eifert, Nour Eddine El Mokhtari, Stephen G. Ellis, Roberto Elosua, James C. Engert, Stephen E. Epstein, Ulf de Faire, Marcus Fischer, Aaron R. Folsom, Jennifer Freyer, Bruna Gigante, Domenico Girelli, Solveig Gretarsdottir, Vilmundur Gudnason, Jeffrey R. Gulcher, Eran Halperin, Naomi Hammond, Stanley L. Hazen, Albert Hofman, Benjamin D. Horne, Thomas Illig, Carlos Iribarren, Gregory T. Jones, J.Wouter Jukema, Michael A. Kaiser, Lee M. Kaplan, John J.P. Kastelein, Kay-Tee Khaw, Joshua W. Knowles, Genovefa Kolovou, Augustine Kong, Reijo Laaksonen, Diether Lambrechts, Karin Leander, Guillaume Lettre, Mingyao Li, Wolfgang Lieb, Patrick Linsel-Nitschke, Christina Loley, Andrew J. Lotery, Pier M. Mannucci, Seraya Maouche, Nicola Martinelli, Pascal P. McKeown, Christa Meisinger, Thomas Meitinger, Olle Melander, Pier Angelica Merlini, Vincent Mooser, Thomas Morgan, Thomas W. Mühleisen, Joseph B. Muhlestein, Thomas Münzel, Kiran Musunuru, Janja Nahrstaedt, Christopher P. Nelson, Markus M. Nöthen, Oliviero Olivieri, Riyaz S. -

Gnb3-/- Rats Were Developed and Similar to Gnb3 -/- Mice Had a Severe Reduction in Rod on Bipolar Cell Signaling
-/- GNB3 RAT: A MODEL FOR Gβ3-ASSOCIATED RETINOPATHY By Marianna Bacellar Teodoro da Silva Galdino A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Comparative Medicine and Integrative Biology- Doctor of Philosophy 2017 ABSTRACT -/- GNB3 RAT: A MODEL FOR Gβ3-ASSOCIATED RETINOPATHY By Marianna Bacellar Teodoro da Silva Galdino The G protein β subunit 3 (Gβ3) is part of the G-protein signaling cascade in retinal ON bipolar cells (BCs), and cone photoreceptors. Knockout of the encoding gene (Gnb3) in mice results in reduced cone photoreceptor sensitivity and marked impairment of both rod and cone ON BC signaling. The latter is revealed as a severe reduction in both the dark- and light-adapted b-wave of the electroretinogram (ERG). Mutations in GNB3 have been recently reported as an apparently stationary condition associated with a variable degree of loss of rod ERG b-wave and the ON BC component of the light-adapted ERG. Gnb3-/- rats were developed and similar to Gnb3 -/- mice had a severe reduction in rod ON bipolar cell signaling. However, in contrast to the mouse model, cone ON BC signaling was relatively well preserved. Application of L-AP4, a blocker of photoreceptor to ON BC signaling, removed the remaining b-wave from the cone ERG confirming it originated from cone ON BCs. Single cell recording from Gnb3-/- retinal slices showed some cone ON BC were functional while rod ON BCs were not functional. Retinal gene therapy are being extensively used as novel treatments to genetic diseases in the retina. -

G Protein Mutations in Endocrine Diseases
European Journal of Endocrinology (2001) 145 543±559 ISSN 0804-4643 INVITED REVIEW G protein mutations in endocrine diseases Andrea Lania, Giovanna Mantovani and Anna Spada Institute of Endocrine Sciences, Ospedale Maggiore IRCCS, University of Milan, Via F. Sforza 35, 20122 Milano, Italy (Correspondence should be addressed to A Spada, Istituto di Scienze Endocrine, Pad. Granelli, Ospedale Maggiore IRCCS, Via Francesco Sforza 35, 20122 Milano, Italy; Email: [email protected]) Abstract This review summarizes the pathogenetic role of naturally occurring mutations of G protein genes in endocrine diseases. Although in vitro mutagenesis and transfection assays indicate that several G proteins have mitogenic potential, to date only two G proteins have been identi®ed which harbor naturally occurring mutations, Gsa, the activator of adenylyl cyclase and Gi2a, which is involved in several functions, including adenylyl cyclase inhibition and ion channel modulation. The gene encoding Gsa (GNAS1) may be altered by loss or gain of function mutations. Indeed, heterozygous inactivating germ line mutations in this gene cause pseudohypoparathyroidism type Ia, in which physical features of Albright hereditary osteodystrophy (AHO) are associated with resistance to several hormones, i.e. PTH, TSH and gonadotropins, that activate Gs-coupled receptors or pseudopseudohypoparathyroidism in which AHO is the only clinical manifestation. Evidence suggests that the variable and tissue-speci®c hormone resistance observed in PHP Ia may result from tissue- speci®c imprinting of the GNAS1 gene, although the Gsa knockout model only in part reproduces the human AHO phenotype. Activating somatic Gsa mutations leading to cell proliferation have been identi®ed in endocrine tumors constituted by cells in which cAMP is a mitogenic signal, i.e. -
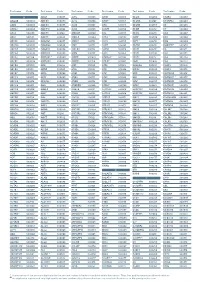
Aagab S00002 Aars S00003 Aars2 S00004 Aass S02483
Test name Code Test name Code Test name Code Test name Code Test name Code Test name Code A ADAR S00053 ALPL S00105 ARSB S00153 BCL10 S02266 C5AR2 S00263 AAGAB S00002 ADCK3 S00054 ALS2 S00106 ARSE * S00154 BCL11A S02167 C5ORF42 S00264 AARS S00003 ADCK4 S00055 ALX3 S00107 ARX S00155 BCL11B S02358 C6 S00265 AARS2 S00004 ADCY10 S02094 ALX4 S00108 ASAH1 S00156 BCOR S00212 C7 S00266 AASS S02483 ADCY3 S02184 AMACR S00109 ASL S00157 BCS1L S00213 C8A S00267 ABAT S02191 ADCY5 S02226 AMELX S02289 ASNS * S02508 BDNF S02509 C8B S00268 ABCA1 S00005 ADGRG1 S00057 AMER1 S00110 ASPA S00158 BDP1 * S00214 C8G S00269 ABCA12 S00006 ADGRG6 S02548 AMH S00111 ASPH S02425 BEAN1 S00215 C8ORF37 S00270 ABCA3 S00007 ADGRV1 S00058 AMHR2 S00112 ASPM S00159 BEST1 S00216 C9 S00271 ABCA4 S00008 ADIPOQ S00059 AMN S00113 ASS1 S00160 BFSP1 S02280 CA2 S00272 ABCA7 S02106 ADIPOR1 * S00060 AMPD1 S02670 ATAD3A * S02196 BFSP2 S00217 CA4 S02303 ABCB11 S00009 ADIPOR2 S00061 AMPD2 S02128 ATCAY S00162 BGN S02633 CA8 S00273 ABCB4 S00010 ADK S02595 AMT S00114 ATF6 S00163 BHLHA9 S00218 CABP2 S00274 ABCB6 S00011 ADNP S02320 ANG S00115 ATIC S02458 BICD2 S00220 CABP4 S00275 ABCB7 S00012 ADSL S00062 ANK1 S00116 ATL1 S00164 BIN1 S00221 CACNA1A S00276 ABCC2 S00013 AFF2 S00063 ANK2 S00117 ATL3 S00165 BLK S00222 CACNA1C * S00277 ABCC6 * S00014 AFG3L2 * S00064 ANKH S00118 ATM S00166 BLM S00223 CACNA1D S00278 ABCC8 S00015 AGA S00065 ANKRD11 * S02140 ATOH7 S02390 BLNK S02281 CACNA1F S00279 ABCC9 S00016 AGBL5 S02452 ANKS6 S00121 ATP13A2 S00168 BLOC1S3 S00224 CACNA1H S00280 ABCD1 * S00017 AGK *