Committee Practical Information Phd Awards
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Digital Soil Map of the World
THE DIGITAL SOIL MAP OF THE WORLD FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Version 3.6, completed January 2003 (C) FAO/UNESCO, 1995 All rights reserved worldwide. Background The present version (3.6) of the digitized Soil Map of the World has been cleaned of errors both in the database and in the lines constituting the digitized map itself. The original map sheets covering the Americas are in bipolar oblique conformal projection. The other sheets, covering Europe, Africa, Asia and Australasia, are based on the Miller oblated stereographic projection; a system consisting of three conformal projections centred on each continent, joined together in a continuous fashion by so-called "fill-in" projections. This allows a complete angular continuity between all sheets. The soil map was prepared using the topographic map series of the American Geographical Society of New York as a base at a nominal scale of 1:5 000 000. The base map comprises sixteen sheets; for the purpose of the Soil Map of the World the information has been redistributed over eighteen sheets in order to obtain sheets of equal size. A nineteenth sheet contains the legend. The digital database is in the Geographic projection. All maps were intersected with a template containing water related features (coastlines, lakes, islands, glaciers and double-lined rivers). This layer was superimposed on the soil map (the information is represented in the FAOSOIL item as: inland WATer, and GLaciers). The Soil Map of the World except for Africa was intersected with the Country Boundaries map from the World Data Bank II (with country boundaries updated to January 1994 at 1:3 000 000 scale), obtained from the US Government. -

Download E-Book (PDF)
OPEN ACCESS International Journal of Livestock Production April-June 2020 ISSN 2141-2448 DOI: 10.5897/IJLP www.academicjournals.org About IJLP The International Journal of Livestock Production (IJLP) is a peer reviewed journal. The journal is published monthly and covers all areas of the subject such as: Selective breeding in animal husbandry, Health effects of animal cruelty, Feed evaluation and feeding, Diet and animal health, Productivity and product composition (milk, meat and eggs), Sustainable livestock management systems, Whole farm management strategies, Animal work, Systems modelling Traceability, Socio-economic consequences of systems, Impact of animals on soil, water, biodiversity. Open Access Policy Open Access is a publication model that enables the dissemination of research articles to the global community without restriction through the internet. All articles published under open access can be accessed by anyone with internet connection. The International Journal of Livestock Production is an Open Access journal. Abstracts and full texts of all articles published in this journal are freely accessible to everyone immediately after publication without any form of restriction. Article License All articles published by International Journal of Livestock Production are licensed under the Creative Commons Attribution 4.0 International License. This permits anyone to copy, redistribute, remix, transmit and adapt the work provided the original work and source is appropriately cited. Citation should include the article DOI. The article license is displayed on the abstract page the following statement: This article is published under the terms of the Creative Commons Attribution License 4.0 Please refer to https://creativecommons.org/licenses/by/4.0/legalcode for details about Creative Commons Attribution License 4.0 Article Copyright When an article is published by in the International Journal of Livestock Production, the author(s) of the article retain the copyright of article. -

Děkanská Zpráva 2014
ČESKÁ ZEMĚDĚLSKÁ UNIVERZITA V PRAZE Fakulta agrobiologie, potravinových a přírodních zdrojů Děkanská zpráva Děkanská zpráva za rok 2014 Děkanská Zpráva Fakulta agrobiologie, potravinových a přírodních zdrojů 2014 za ROK 2014 titulni2014_v2_.indd 1 24.3.2015 8:54:35 DĚKANSKÁ ZPRÁVA ZA ROK 2014 Děkanská zpráva 3 za rok 2014 OBSAH I. ORGANIZAČNÍ STRUKTURA FAKULTY 7 II. STUDIJNÍ A PEDAGOGICKÁ ČINNOST 8 III. VĚDECKOVÝZKUMNÁ ČINNOST 12 IV. MEZINÁRODNÍ VZTAHY 13 V. OBLAST ROZVOJE FAKULTY 17 VI. OBLAST PERSONÁLNÍHO ZAJIŠTĚNÍ A KVALIFIKAČNÍ STRUKTURA FAKULTY 18 KATEDRA AGROEKOLOGIE A BIOMETEOROLOGIE 21 KATEDRA AGROENVIRONMENTÁLNÍ CHEMIE A VÝŽIVY ROSTLIN 37 KATEDRA BOTANIKY A FYZIOLOGIE ROSTLIN 55 KATEDRA GENETIKY A ŠLECHTĚNÍ 63 KATEDRA CHEMIE 71 KATEDRA KVALITY ZEMĚDĚLSKÝCH PRODUKTŮ 81 KATEDRA MIKROBIOLOGIE, VÝŽIVY A DIETETIKY 89 KATEDRA OCHRANY ROSTLIN 101 KATEDRA OBECNÉ ZOOTECHNIKY A ETOLOGIE 111 KATEDRA PEDOLOGIE A OCHRANY PŮD 123 KATEDRA PÍCNINÁŘSTVÍ A TRÁVNÍKÁŘSTVÍ 139 KATEDRA ROSTLINNÉ VÝROBY 147 KATEDRA SPECIÁLNÍ ZOOTECHNIKY 169 KATEDRA VETERINÁRNÍCH DISCIPLÍN 185 KATEDRA VODNÍCH ZDROJŮ 197 KATEDRA ZAHRADNICTVÍ 205 KATEDRA ZAHRADNÍ A KRAJINNÉ ARCHITEKTURY 211 KATEDRA ZOOLOGIE A RYBÁŘSTVÍ 219 DEMONSTRAČNÍ A EXPERIMENTÁLNÍ PRACOVIŠTĚ 235 4 Fakulta agrobiologie, potravinových a přírodních zdrojů Děkanská zpráva 5 za rok 2014 Vážené kolegyně, vážení kolegové, vítám Vás nad Děkanskou zprávou 2014, tradičním materiálem hodnotícím aktivity realizované vedením fakulty a Vámi, pedagogickými, technickými i administrativními pracovníky Fakulty agrobiologie, potravinových a přírodních zdrojů ČZU v Praze. Tato zpráva ve své první části souhrnně prezentuje výčet hlavních činností za minulý kalendářní rok, v druhé části pak stav a shrnutí aktivit jednotlivých kateder fakulty a jejich součástí. Máte v rukou publikaci, která Vám všem nabízí jedinečnou možnost srovnání našich výsledků v časové řadě a především porovnat aktivity jednotlivých pracovišť a zhodnotit jejich rozvoj či stagnaci. -

Diplomka-Nova Stranky
Univerzita Palackého v Olomouci Přírodov ědecká fakulta Katedra rozvojových studií Jitka ŠÁDKOVÁ ROZVOJOVÉ INTERVENCE ČESKÉ REPUBLIKY A ZEMÍ OECD V ANGOLE Diplomová práce Vedoucí práce: Mgr. Zden ěk Opršal Olomouc 2009 Prohlašuji, že jsem diplomovou práci vypracovala samostatn ě a veškeré použité prameny jsem řádn ě citovala a uvedla v seznamu použitých zdroj ů. V Olomouci 10. kv ětna 2009 …………………………… Podpis Děkuji Mgr. Zde ňku Opršalovi za cenné rady, vst řícný p řístup a odborné vedení mé diplomové práce. Vysoká škola: Univerzita Palackého Fakulta: P řírodov ědecká Katedra: Geografie Školní rok: 2006/07 ZADÁNÍ DIPLOMOVÉ PRÁCE student Jitka ŠÁDKOVÁ obor Mezinárodní rozvojová studia Název práce: Rozvojové intervence České republiky a zemí OECD v Angole Development Intervention of the Czech Republic and OECD Countries in Angola Zásady pro vypracování: Cílem diplomové práce je monitoring a analýza rozvojových intervencí České republiky a zemí OECD v Angole. Bude provedena charakteristika Angoly, její historie a sou časné problémy. Bude sledována česká rozvojová pomoc a rozvojová pomoc zemí OECD Angole. Budou zmín ěny priority, sektorové zam ěř ení a projekty České republiky a jednotlivých zemí OECD v Angole a provedena komparace rozvojové pomoci. V rámci diplomové práce bude také v ěnována pozornost angolské národnostní menšin ě v České republice. Struktura práce: 1. Úvod 2. Cíl práce 3. Metody zpracování 4. Charakteristika Angoly 5. Rozvojové intervence České republiky v Angole 6. Rozvojové intervence zemí OECD v Angole 7. Komparace rozvojové pomoci 8. Angolská národnostní menšina v České republice 9. Shrnutí (v angli čtin ě) 10. Záv ěr Diplomová práce bude zpracována v t ěchto kontrolovaných etapách: rešerše literárních pramen ů a metodika listopad 2006 – leden 2007 kapitola č. -

Genetics and Immunity of Indigenous Chicken in Kenya
GENETICS AND IMMUNITY OF INDIGENOUS CHICKEN IN KENYA JOEL ONYANGO KHOBONDO A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT FOR THE REQUIREMENT OF THE AWARD OF THE DOCTOR OF PHILOSOPHY DEGREE IN ANIMAL SCIENCE OF EGERTON UNIVERSITY EGERTON UNIVERSITY OCTOBER 2018 DECLARATION AND RECOMMENDATION DECLARATION This thesis is my original work and has not, wholly or in part, been presented for the award of a degree in any University. Signature: ……………………………… Date: ……………………….. Mr. Ir. Joel Onyango Khobondo KD111/13629/14 RECOMMENDATION This thesis is the candidate’s original work and has been prepared with our guidance and assistance; it has been submitted with our approval as the official university supervisors. Signature: ……………………………… Date: ………………………. Prof. Alexander K. Kahi. Department of Animal Sciences Egerton University. Signature: ……………………………… Date: ………………………. Dr. Chrilukovian B. Wasike. Department of Animal Science Maseno University. Signature: ……………………………… Date: ………………………. Dr. Ramadhan S. Mwakubambanya. Department of Biochemistry and Molecular Biology Egerton University. i COPY RIGHT ©2018, Khobondo J.O No part or whole of this thesis may be reproduced or transmitted in any form or means such as electronic, mechanical including photocopying, recording or any information storage and retrieval system without prior permission of the author or Egerton University on behalf of the author. All rights reserved ii DEDICATION This work is dedicated to all stakeholders in indigenous chicken value chain, my family and supervisors for their unmatched support. iii ACKNOWLEDGEMENT I am very grateful to the Almighty God for the good health, mental stability, physical strength and gift of life during the whole PhD programme. The development of this thesis could not be possible without the support of a number of personalities and institutions. -
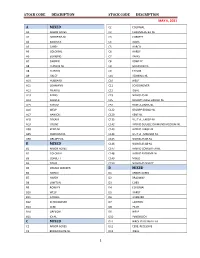
Stock Codes Numerical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C2 COLONIAL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 ANDREWS-NL C3 CORBETT A2 BABCOCK C4 DAVIS A3 CAREY C5 HARCO A5 COLONIAL C6 HARDY A6 EURIBRID C7 PARKS A7 GARBER C8 ROWLEY A8 H AND N-NL C9 GUILFORD-NL A8 H AND N C9 TATUM A9 HALEY C10 HENNING-NL A10 HUBBARD C10 WELP A11 LOHMANN C11 SCHOONOVER A12 MERRILL C12 IDEAL A13 PARKS C19 NICHOLAS-NL A14 SHAVER C35 ORLOPP LARGE BROAD-NL A15 TATUM C57 ROSE-A-LINDA-NL A16 WELP C122 ORLOPP BROAD-NL A17 HANSON C129 KENT-NL A18 DEKALB C135 B.U.T.A., LARGE-NL A19 HYLINE C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A38 KENT-NL C143 HYBRID LARGE-NL A45 MARCUM-NL C144 B.U.T.A., MEDIUM-NL A58 ORLOPP-NL C145 NICHOLAS 85-NL B MIXED C146 NICHOLAS 88-NL B1 ARBOR ACRES C147 HYBRID CONVERTER-NL B2 COLONIAL C148 HYBRID EXTREME-NL B3 CORBETT C149 MIXED B4 DAVIS C150 NICHOLAS SELECT B5 DEKALB WARREN D MIXED B6 HARCO D1 ARBOR ACRES B7 HARDY D2 BRADWAY B8 LAWTON D3 COBB B9 ROWLEY D4 COLONIAL B10 WELP D5 HARDY B11 CARGILL D6 HUBBARD B12 SCHOONOVER D7 LAWTON B13 CEBE D8 PILCH B14 OREGON D9 WELP B15 IDEAL D10 PENOBSCOT C MIXED D11 WROLSTAD SMALL-NL C1 ARBOR ACRES D11 CEBE, RECESSIVE C2 BROADWHITE-NL D12 IDEAL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N14 OLD ENGLISH, WHITE E MIXED N15 OLD ENGLISH, BLACK E1 COLONIAL N16 OLD ENGLISH, SPANGLED E2 HUBBARD N17 PIT E3 BOURBON, RED-NL N18 OLD ENGLISH E3 ROWLEY N19 MODERN E4 WELP N20 PIT, WHITE HACKLE E5 SCHOONOVER N21 SAM BIGHAM E6 CEBE N22 MCCLANHANS H MIXED N23 CLIPPERS H1 ARBOR ACRES N24 MINER BLUES H2 -

VIV MEA 2018 Closing Press Release VIV MEA Second Edition Confirms
[Utrecht, 6 March 2018] VIV MEA 2018 Closing Press Release VIV MEA second edition confirms its regional hub status for industry leaders from feed to food Good reviews from exhibitors and visitors has set the seal on a successful second edition of regional feed-to-food trade show VIV MEA. Their comments about the February 2018 event confirmed the value of having a truly professional and international show in the region that extends from the Middle East and Africa to Iran and the countries of the Indian sub-continent. Bigger show welcomed high-quality visitors Held in the United Arab Emirates between 5th-7th February, VIV MEA 2018 was already going to be bigger than the 2016 edition because the show had spread into a fourth hall at the Abu Dhabi National Exhibition Centre (ADNEC) to satisfy the extra demand for stands. Final statistics for the 2018 show confirm that it was also bigger on attendance figures. Its 3-day total of 6,660 visits represented a 6.7% increase compared with the first edition. Evidently the expanded exhibition this time was a factor in attracting more visitors, even if it impacted on traffic density around the stands at times by spreading the attendance across more square metres of space. But the key point about the attendees was their quality. A survey conducted during the three weeks after the show has found a satisfaction score of 7.3 out of 10 from exhibitors and an even higher rating of 8.3 from visitors. Many exhibitors commented on the high quality of the VIV MEA visitors in 2018, confirming the evidence from registration details for visitor job title, function and decision-making power. -

Exhibitor Country Hall Booth Sector 1 1/5 Veterinary Medicine Company
Exhibitor Country Hall Booth Sector 1 1/5 Veterinary Medicine Company - TRAVETCO Viet Nam H106 254 VIVA Animal Health 2 A & S Thai Works Co., Ltd Thailand H098 4053 VIVA Processing / Handling 3 A.W.P. SRL Italy H103 2010 VIVA Feed ingredients & additives 4 A/B Technologies Int'l Inc. United States H102 2110 VIVA Feed ingredients & additives 5 AAT-Freezing Austria H098 4559 VIVA Logistics / Refrigeration 6 AB Kauno Grüdai Lithuania H105 917 VIVA Feed ingredients & additives 7 AB VISTA ASIA PTE LTD Singapore H103 1820 VIVA Feed ingredients & additives 8 ABRA - Brazilian Renderers Association Brazil H102 2200 VIVA Feed ingredients & additives 9 Absortech (Siam) Co., Ltd. Thailand H098 4330 VIVA Logistics / Refrigeration 10 ACG Products United States H102 2240 VIVA Feed ingredients & additives 11 ACO Funki A/S Denmark H100 3123 VIVA Farm Production 12 ACTINI SAS France H098 4440 VIVA Processing / Handling 13 AdBiotech Co., Ltd South Korea H105 1062 VIVA Animal Health 14 ADDCON Asia Ltd. Hong Kong H103 1821 VIVA Feed ingredients & additives 15 ADDiCAN Animal Nutrition United States H102 2475 16 Ademco 2 GmbH Germany H099 3675 VIVA Farm Production 17 Adifo N.V. Belgium H102 2557 VIVA IT service providers 18 Adisseo / Nutriad International NV Belgium H103 1901 VIVA Feed ingredients & additives 19 Adisseo Asia Pacific Pte. Ltd Singapore H103 1800 VIVA Feed ingredients & additives 20 ADM Animal Nutrition, Inc. United States H102 2341 VIVA Feed ingredients & additives 21 Advanced Enzyme Technologies Limited India H103 2015 VIVA Feed ingredients & additives 22 Aerox B.V. Netherlands H102 2416 VIVA Feed/CropTech-FeedTech 23 Afoheat bvba Belgium H098 4665 VIVA (VIV Asia) 24 Ag Growth International Canada H105 1087 VIVA (VIV Asia) 25 AGCO GSI (Malaysia) Sdn. -

Market Study on the Intensive Animal Production Chain (Pigs & Poultry)
Market study on the intensive animal production chain (pigs & poultry) in the Czech Republic Prepared by: Martin Sedláček Director Profi Press ltd. Prague, March 2006 1 Contents Part 1: Pork production chain Page 1. Primary production sector – present situation and future developments 5 1.1. Core information 5 1.2. Pig numbers 6 1.3. Pig breeders 6 1.4. Genetics and breeding 8 1.5. Breeding economics 9 1.6. Future developments 11 1.7. Animal healt status 11 2. Slaughter/processing sector: present situation and future developments 12 2.1. Core information 12 2.2. Processing of pigs 12 2.3. Ownership structure of the main processing capacities 13 2.4. Outlook 13 3. Consumption: present situation and future developments 15 3.1. Present situation 15 3.2. Future developments 15 4. Foreign trade: present situation and future developments 16 4.1. Core information 16 4.2. Commodity trade 16 4.3. Outlook 18 5. Distribution/retail: present situation and future developments 19 5.1. Core information 19 5.2. Outlook 20 6. Supply sector: present situation and future developments 21 6.1. Genetics 21 6.2. Housing and housing equipment 21 6.3. Pig nutrition 21 6.4. Veterinary medicines 22 7. Foreign involvement/investment 23 8. Institutional sector 24 9. SWOT 26 10. Possibilities for Dutch agribusiness for trade/investment 27 Annex 1. Description of selected farms 28 Annex 2. List of contacts (addresses of major producers, slaughterhouses, 31 processors, importers, traders, distribution, supply companies, institutional sector) 2 Part 2: Poultry production chain Page 1. Primary production sector – present situation and future developments 48 1.1. -

2020-01-06 Exhibitor List
Preliminary exhibitors list - VIV MEA 2020 # Exhibitor Country Booth Sector 1 Emre Pilic Ltd. Sti Turkey 1.0U015 VIV MEA Breeding / hatching 2 EmTech Hatchery SystemsUnited Limited Kingdom 1.0U010 VIV MEA Breeding / hatching 3 EUROFEED TECHNOLOGIESItaly S.p.A. 9.P066 VIV MEA Feed ingredients & additives 4 Europack Slovakia 9.P037 VIV MEA Processing / Handling 5 Eurotecs Çelik San. Tic. LtdTurkey Sti. 7.C024 VIV MEA Farm Production 6 Evonik Nutrition & Care GmbHGermany NC-SD 7.c044 VIV MEA Feed ingredients & additives 7 EW Nutrition GmbH Germany 7.D062 VIV MEA Feed ingredients & additives 8 Exafan S.A. Spain 8.H015 VIV MEA Farm Production 9 Expert-Medical Ltd. Hungary 1.0T041 VIV MEA Animal health 10 Facco S.P.A. Italy 7.E011 VIV MEA Farm Production 11 Fancom B.V. Netherlands 7.D012 VIV MEA Farm Production 12 Farm Faes - Tecnovit Spain 8.L055 VIV MEA Feed ingredients & additives 13 Federal State Enterprise RussianShchelkovo Federation Biocombinat9.N049 VIV MEA Animal health 14 Feepro Lebanon 9.N025 VIV MEA Feed/ CropTech-FeedTech 15 FF Chemicals BV Netherlands 7.A061 VIV MEA Feed ingredients & additives 16 FGBI (ARRIAH) Russian Federation9.N057 VIV MEA Animal health 17 Fiem Srl Italy 1.0V002 VIV MEA Breeding / hatching 18 FLEXY S.r.l. Italy 7.C015 VIV MEA Farm Production 19 Food Export- Midwest United States 0.8G051 20 Food Forward Uruguay S.A.Uruguay 8.G034 VIV MEA Logistics / Refrigeration 21 Foodmate B.V. Netherlands 9.T010 VIV MEA Processing / Handling 22 Fornazor International, Inc.United States 0.8H054 23 Frigortec GmbH Germany 9.N031 VIV MEA Feed/ CropTech-FeedTech 24 G. -

Recruiting Assemblages of Contract Labour in Fernando Pó and the Gulf of Guinea, 1858–1979
Humboldt-Universität zu Berlin Dissertation Touts and Despots: Recruiting Assemblages of Contract Labour in Fernando Pó and the Gulf of Guinea, 1858–1979 zur Erlangung des akademischen Grades doctor philosophiae (Dr. phil.) Kultur-, Sozial- und Bildungswissenschaftlichen Fakultät Enrique Martino Präsident der Humboldt-Universität zu Berlin Prof. Dr. Jan-Hendrik Olbertz Dekanin der Kultur-, Sozial- und Bildungswissenschaftlichen Fakultät Prof. Dr. Julia von Blumenthal Gutachter: 1. Prof. Dr. Andreas Eckert 2. Prof. Dr. Alexander Keese Datum der Einreichung: 18 November 2015 Tag der mündlichen Prüfung: 22 April 2016 1 2 Abstract This dissertation follows Fernando Pó’s labour recruiters wherever they went— between the 1860s and 1920s recruiters traversed the entirety of the Gulf of Guinea and enlisted mostly Kru from Liberia and Fang from Rio Muni, Cameroon and Gabon; between the 1930s to 1960s they gathered particularly around the Bight of Biafra and brought an unprecedented number of contract workers into the island’s booming cacao plantations, mostly Igbos and Ibibios from south-eastern Nigeria. Recruiters tended to appear in a modality that I will describe and theorize as ‘touts’. They operated almost exclusively with an excess of language and money—deceit and informal advances. They operated ‘outside’ the law and the regulated, yet it was only the shape of the contract on Fernando Pó—forced, long and irrevocable—that allowed recruiters to deploy their techniques. Recruiters created and relayed a series of wholly impermissible twists: quasi-enslavement through fraud that was a form of kidnapping, quasi-debt bondage with informal wage advances enabled by the contracts, and even a movement of really quite free but fugitive labour across borders and work-sites. -

No Company Country Booth Sector 1 4B Asia Pacific Company Limited Vietnam G20 Feed / Croptech
ILDEX VIETNAM 2020 EXHIBITOR LIST As of 20/Nov/2019 - Subject to change NO COMPANY COUNTRY BOOTH SECTOR 1 4B ASIA PACIFIC COMPANY LIMITED VIETNAM G20 FEED / CROPTECH - FEEDTECH 2 A&S THAI WORK COMPANY LIMITED THAILAND K4 FEED / CROPTECH - FEEDTECH 3 AB VISTA ASIA PTE LTD UK BC13 FEED / INGREDIENTS & ADDITIVES 4 ABRA - BRAZILIAN ANIMAL RECYCLING ASSOCIATION BRAZIL AB13 FEED / INGREDIENTS & ADDITIVES 5 AG GROWTH INTERNATIONAL INC CANADA H28 FEED / CROPTECH - FEEDTECH 6 AGRINOVATE COMPANY LIMITED THAILAND L1 FARM PRODUCTION 7 AGROLOGIC LIMITED ISRAEL HJ9 FARM PRODUCTION 8 AGROMED AUSTRIA GMBH AUSTRIA D21 FEED / INGREDIENTS & ADDITIVES 9 ANGEL YEAST COMPANY LIMITED CHINA AB17 FEED / INGREDIENTS & ADDITIVES 10 ANHUI XINYUAN PACKING TECHNOLOGY COMPANY LIMITED CHINA K1 FARM PRODUCTION 11 ARDES GROUP FRANCE G28 FARM PRODUCTION 12 ASIA PACIFIC IMPORT EXPORT JOINT STOCK COMPANY (APC) VIETNAM BC25 FARM PRODUCTION 13 ASIAN AGRIBUSINESS MEDIA PTE LIMITED SINGAPORE M27 KNOWLEDGE TRANSFER / CONSULTANCY 14 ASTINO AGRO-HOUSE MULTI SYSTEM SDN BHD MALAYSIA FG17 FARM PRODUCTION 15 AVIAGEN ASIA PACIFIC LIMITED UNITED KINGDOM FG17 BREEDING / HATCHING 16 AXIOM INTERNATIONAL FRANCE BC25 BREEDING / HATCHING 17 AZA INTERNATIONAL SRL ITALY H2 FARM PRODUCTION 18 AZIM-TAV POULTRY EQUIPMENT COMPANY TURKEY L3 FARM PRODUCTION 19 B.H.N COMPANY LIMITED VIETNAM LM13 FARM PRODUCTION 20 BAN HANG XANH COMPANY LIMITED VIETNAM E23 ANIMAL HEALTH 21 BEHN MEYER GROUP (VIETNAM) GERMANY BC13 FEED / INGREDIENTS & ADDITIVES 22 BEIJING YAHE NUTRITIVE HIGH TECH COMPANY LIMITED CHINA