Statistical Methods for Biosecurity Monitoring and Surveillance Author(S) / Address (Es) David Fox, University of Melbourne
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government Gazette of the STATE of NEW SOUTH WALES Number 168 Friday, 30 December 2005 Published Under Authority by Government Advertising and Information
Government Gazette OF THE STATE OF NEW SOUTH WALES Number 168 Friday, 30 December 2005 Published under authority by Government Advertising and Information Summary of Affairs FREEDOM OF INFORMATION ACT 1989 Section 14 (1) (b) and (3) Part 3 All agencies, subject to the Freedom of Information Act 1989, are required to publish in the Government Gazette, an up-to-date Summary of Affairs. The requirements are specified in section 14 of Part 2 of the Freedom of Information Act. The Summary of Affairs has to contain a list of each of the Agency's policy documents, advice on how the agency's most recent Statement of Affairs may be obtained and contact details for accessing this information. The Summaries have to be published by the end of June and the end of December each year and need to be delivered to Government Advertising and Information two weeks prior to these dates. CONTENTS LOCAL COUNCILS Page Page Page Albury City .................................... 475 Holroyd City Council ..................... 611 Yass Valley Council ....................... 807 Armidale Dumaresq Council ......... 478 Hornsby Shire Council ................... 614 Young Shire Council ...................... 809 Ashfi eld Municipal Council ........... 482 Inverell Shire Council .................... 618 Auburn Council .............................. 484 Junee Shire Council ....................... 620 Ballina Shire Council ..................... 486 Kempsey Shire Council ................. 622 GOVERNMENT DEPARTMENTS Bankstown City Council ................ 489 Kogarah Council -

Pittwater and Warringah (Part) 1 Local Government Boundaries Commission
Local Government Boundaries Commission 1. Summary of Local Government Boundaries Commission comments The Boundaries Commission has reviewed the Delegate’s Report on the proposed merger of Pittwater Council and part of Warringah Council to determine whether it shows the legislative process has been followed and the Delegate has taken into account all the factors required under the Local Government Act 1993 (the Act). The Commission has assessed that: the Delegate’s Report shows that the Delegate has undertaken all the processes required by section 263 of the Act, the Delegate’s Report shows that the Delegate has adequately considered all the factors required by section 263(3) of the Act, with the exception of the factors listed under subsections 263(3)(e2) (employment impacts) and 263(3)(e5) (diverse communities), and the Delegate’s recommendation in relation to the proposed merger is supported by the Delegate’s assessment of these factors. 2. Summary of the merger proposal On 6 January 2016, the Minister for Local Government referred a proposal to merge the local government areas of Pittwater Council and part of Warringah Council to the Acting Chief Executive of the Office of Local Government for examination and report under the Act. The following map shows the proposed new council area (shaded in green). Proposed merger of Pittwater and Warringah (part) 1 Local Government Boundaries Commission The proposal would have the following impacts on population across the two councils. Council 2016 2031 Pittwater Council 63,900 77,600 Warringah Council (part) 77,343 89,400 Merged entity 141,243 167,000 Source: NSW Department of Planning & Environment, 2014 NSW Projections (Population, Household and Dwellings), and NSW Government, January 2016 ,Merger Proposal: Pittwater Council and Warringah Council (part), p8. -

17 December 2019
MINUTES OF THE ORDINARY MEETING OF COUNCIL commencing at 5.02pm on TUESDAY 17 DECEMBER 2019 Council Chambers 11 Manning Street, KIAMA NSW 2533 MINUTES OF THE ORDINARY MEETING 17 DECEMBER 2019 Contents MINUTES OF THE ORDINARY MEETING OF THE COUNCIL OF THE MUNICIPALITY OF KIAMA HELD IN THE COUNCIL CHAMBERS, KIAMA ON TUESDAY 17 DECEMBER 2019 AT 5.02PM PRESENT: Mayor – Councillor M Honey, Deputy Mayor – Councillor A Sloan, Councillors M Brown, N Reilly, K Rice, W Steel, D Watson, M Way and M Westhoff IN ATTENDANCE: General Manager, Acting Director Environmental Services, Acting Director Corporate and Commercial Services, Acting Director Engineering and Works and Director Blue Haven 1 Apologies 1 APOLOGIES Nil. 2 Acknowledg ement of Traditional owners 2 ACKNOWLEDGEMENT OF TRADITIONAL OWNERS The Mayor declared the meeting open and acknowledged the traditional owners: “I would like to acknowledge the traditional owners of the Land on which we meet, the Wadi Wadi people of the Dharawal nation, and pay my respect to Elders past and present.” 3.1 Or dinar y C ouncil on 19 N ovember 2019 3 Confirmati on of Minutes of Pr evious M eeting 3.1 ORD INAR Y C OUNCIL ON 19 N OVEM BER 2019 3 CONFIRMATION OF MINUTES OF PREVIOUS MEETING 3.1 Ordinary Council on 19 November 2019 19/464OC Resolved that the Minutes of the Ordinary Council Meeting held on 19 November 2019 be received and accepted. (Councillors Way and Steel) For: Councillors Brown, Honey, Reilly, Rice, Sloan, Steel, Watson, Way and Westhoff Against: Nil Kiama Municipal Council Page 2 MINUTES OF -

Hyde Park Management Plan
Hyde Park Reserve Hartley Plan of Management April 2008 Prepared by Lithgow City Council HYDE PARK RESERVE HARTLEY PLAN OF MANAGEMENT Hyde Park Reserve Plan of Management Prepared by March 2008 Acknowledgements Staff of the Community and Culture Division, Community and Corporate Department of Lithgow City Council prepared this plan of management with financial assistance from the NSW Department of Lands. Valuable information and comments were provided by: NSW Department of Lands Wiradjuri Council of Elders Gundungurra Tribal Council members of the Wiradjuri & Gundungurra communities members of the local community and neighbours to the Reserve Lithgow Oberon Landcare Association Central Tablelands Rural Lands Protection Board Lithgow Rural Fire Service Upper Macquarie County Council members of the Hartley District Progress Association Helen Drewe for valuable input on the flora of Hyde Park Reserve Royal Botanic Gardens Sydney Centre for Plant Biodiversity Research, Canberra Tracy Williams - for valuable input on Reserve issues & uses Department of Environment & Conservation (DECC) NW Branch Dave Noble NPWS (DECC) Blackheath DECC Heritage Unit Sydney Photographs T. Kidd This Hyde Park Plan of Management incorporates a draft Plan of Management prepared in April 2003. Lithgow City Council April 2008 2 HYDE PARK RESERVE HARTLEY PLAN OF MANAGEMENT FOREWORD 6 EXECUTIVE SUMMARY 6 PART 1 – INTRODUCTION 7 1.0 INTRODUCTION 8 1.1 PURPOSE OF A PLAN OF MANAGEMENT 8 1.2 LAND TO WHICH THE PLAN OF MANAGEMENT APPLIES 9 1.3 GENERAL RESERVE -
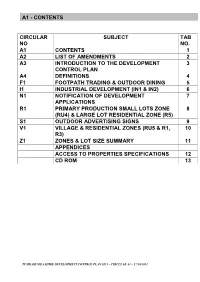
Contents Circular No Subject Tab No
A1 - CONTENTS CIRCULAR SUBJECT TAB NO NO. A1 CONTENTS 1 A2 LIST OF AMENDMENTS 2 A3 INTRODUCTION TO THE DEVELOPMENT 3 CONTROL PLAN A4 DEFINITIONS 4 F1 FOOTPATH TRADING & OUTDOOR DINING 5 I1 INDUSTRIAL DEVELOPMENT (IN1 & IN2) 6 N1 NOTIFICATION OF DEVELOPMENT 7 APPLICATIONS R1 PRIMARY PRODUCTION SMALL LOTS ZONE 8 (RU4) & LARGE LOT RESIDENTIAL ZONE (R5) S1 OUTDOOR ADVERTISING SIGNS 9 V1 VILLAGE & RESIDENTIAL ZONES (RU5 & R1, 10 R3) Z1 ZONES & LOT SIZE SUMMARY 11 APPENDICES ACCESS TO PROPERTIES SPECIFICATIONS 12 CD ROM 13 TUMBARUMBA SHIRE DEVELOPMENT CONTROL PLAN 2011 – CIRCULAR A1 – 27/10/2011 A2 – LIST OF AMENDMENTS Objectives:- Date of adoption of original plan and date when plan comes into force • To identify the process for amending the DCP and providing for public participation This plan was exhibited for public comment in accordance with the Environmental Planning and • Assessment Act 1979 and Regulations. Council To update on amendments to the th Tumbarumba Shire Development Control Plan adopted this plan on the 27 October, 2011 2011 Subsequent amendments to the plan are listed below. • To identify the date of adoption of the DCP by Council and subsequent amendments th This plan came into force as of the 25 April, 2012 (being the date of public notice in the local newspaper in accordance with Clause 21 of the Environmental Planning and Assessment Regulations 2000) Purpose of Amendment Circular Amended Date Amendment effective (i.e. public notice - Clause 21 of EPA Regs) Amendments to Tumbarumba Shire Development Control Plan 2011 Where Council resolves to prepare draft circulars as an amendment to the Tumbarumba Shire Development Control Plan 2011 these must be exhibited for a minimum period of 28 days. -

Gloucester, Greatlakes and Greater Taree
Local Government Boundaries Commission 1. Summary of Local Government Boundaries Commission comments The Boundaries Commission has reviewed the Delegate’s Report on the proposed merger of Gloucester Shire Council, Great Lakes Council, and Greater Taree City Council to determine whether it shows the legislative process has been followed and the Delegate has taken into account all the factors required under the Local Government Act 1993 (the Act). The Commission has assessed that: the Delegate’s Report shows that the Delegate has undertaken all the processes required by section 263 of the Act, the Delegate’s Report shows that the Delegate has adequately considered all the factors required by section 263(3) of the Act with the exception of the factors listed under subsections 263(3)(e1) (service delivery) and 263(3)(e5) (diverse communities), and the Delegate’s recommendation in relation to the proposed merger is supported by the Delegate’s assessment of the factors. 2. Summary of the merger proposal On 8 March 2016 the Minister for Local Government referred a proposal to merge the local government areas of Gloucester, Great Lakes and Greater Taree to the Acting Chief Executive of the Office of Local Government for examination and report under the Act. The following map shows the proposed new council area (shaded in green). Proposed merger of Gloucester, Great Lakes and Greater Taree 1 Local Government Boundaries Commission The proposal would have the following impacts on population across the three councils. Council 2016 2031 Gloucester Shire Council 5,000 4,850 Great Lakes Council 36,700 38,500 Greater Taree City Council 49,450 51,900 New Council 91,150 95,250 Source: NSW Department of Planning & Environment, 2014 NSW Projections (Population, Household and Dwellings). -

Communication Licence Rent
Communication licences Fact sheet Communication licence rent In November 2018, the NSW Premier had the Independent Pricing and Regulatory Tribunal (IPART) undertake a review of Rental arrangements for communication towers on Crown land. In November 2019, IPART released its final report to the NSW Government. To provide certainty to tenure holders while the government considers the report, implementation of any changes to the current fee structure will apply from the next renewal or review on or after 1 July 2021. In the interim, all communication tenures on Crown land will be managed under the 2013 IPART fee schedule, or respective existing licence conditions, adjusted by the consumer price index where applicable. In July 2014, the NSW Government adopted all 23 recommendations of the IPART 2013 report, including a rental fee schedule. Visit www.ipart.nsw.gov.au to see the IPART 2013 report. Density classification and rent calculation The annual rent for communication facilities located on a standard site depends on the type of occupation and the location of the facilities. In line with the IPART 2013 report recommendations, NSW is divided into four density classifications, and these determine the annual rent for each site. Table 1 defines these classifications. Annexure A further details the affected local government areas and urban centres and localities (UCLs) of the classifications. Figure 1 shows the location of the classifications. A primary user of a site who owns and maintains the communication infrastructure will incur the rent figures in Table 2. A co-user of a site will be charged rent of 50% that of a primary user. -

Council Decision Making and Independent Panels
The Henry Halloran Trust Research Report Council Decision Making and Independent Panels Yolande Stone A Practitioner-in-Residence Project A review of the Evolution of Panels and their Contribution to Improving Development Assessment in NSW ISBN: 978-0-9925289-1-1 ACKNOWLEDGEMENTS This material was produced with funding from Henry Halloran Trust at the University of Sydney. The University gratefully acknowledges the important role of the Trust in promoting scholarship, innovation and research in town planning, urban development and land management. The University of Sydney, through the generous gift of Warren Halloran, has established the Henry Halloran Trust in honour of Henry Halloran, who was an active advocate for town planning in the first half of the twentieth century. He introduced and implemented new concepts of town planning in the many settlements he established, as part of h is contribution to nation building. The objective of the trust is to promote scholarship, innovation and research in town planning, urban development and land management. This will be achieved through collaborative, cross- disciplinary and industry-supported research that will support innovative approaches to urban and regional policy, planning and development issues. The Trust’s ambition is to become a leading voice and advocate for the advancement of liveable cities, thriving urban communities and sustainable development. For further information: http://www.sydney.edu.au/halloran I would also like to acknowledge and thank Professor Peter Phibbs Director, Henry Halloran Trust and Dr Michael Bounds, Coordinator of the Practitioner in Residence Program, Henry Halloran Trust for their guidance and support. I would also like to thank council staff, panel members and development assessment experts who provided valuable input into my research. -

Extract from Register of Indigenous Land Use Agreements
Extract from Register of Indigenous Land Use Agreements NNTT number NIA1998/001 Short name Tumut Brungle Area Agreement ILUA type Area Agreement Date registered 21/06/1999 State/territory New South Wales Local government region Gundagai Shire Council, Tumbarumba Shire Council, Tumut Shire Council, Holbrook Shire Council, Wagga Wagga, Yarrowlumla Shire Council, Yass Shire Council Description of the area covered by the agreement The agreement covers an area of approximately 8500 sq km. It’s external boundary (described in detail below) runs approximately from Coolac on the Hume Highway east to Lake Burrinjuck (north east of Wee Jasper); south along the Brindabella and Fiery Ranges to near Yarrangobilly Caves on the Snowy Mountains Highway, south west to the Murray River near Tintaldra; then along the Murray River to Jingellic; and then generally north towards Gundagai and on to Coolac. Description of the area covered by the Agreement : Clause 1.1.2 of the agreement states: "Deed Area" - means the area of land set out in the plan `and description set out at Schedule 1. Schedule 1 of the agreement contains a gazettal notice of the constitution of the Brungle Tumut Local Aboriginal Land Council Area dated 2 February 1984, set out below: BRUNGLE TUMUT LOCAL ABORIGINAL LAND COUNCIL AREA Commencing at the junction of the generally south-eastern boundary of the Parish of Jingellec East with the boundary between the States of New South Wales and Victoria: and bounded thence by the latter boundary generally south-easterly to the Tooma River; by that -

Community Engagement Handbook to You on Behalf of the NSW Government and Our Partners
COMMUNITY ENGAGEMENT COMMUNITY ENGAGEMENT COMMUNITY ENGAGEMENT IN THE NSW PLANNING SYSTEM www.iplan.nsw.gov.au/engagement/ IN THE NSW PLANNING SYSTEM in partnership with www.iplan.nsw.gov.au/engagement/ Prepared for PlanningNSW by Elton Consulting COMMUNITY ENGAGEMENT IN THE NSW PLANNING SYSTEM www.iplan.nsw.gov.au/engagement/ PlanningNSW in partnership with NSW Department of Local Government Lgov NSW Institute of Public Administration Australia (NSW Division) Planning Institute of Australia (NSW Division) International Association for Public Participation NSW Premier’s Department Prepared for PlanningNSW by Elton Consulting © Crown copyright 2003 Department of Planning Henry Deane Building 20 Lee Street Sydney, NSW, Australia 2000 www.planning.nsw.gov.au Published February 2003 ISBN 0 7347 0403 8 Pub no. 03-034A Disclaimer. While every reasonable effort has been made to ensure that this document is correct at the time of printing, the State of New South Wales, its agents and employees, disclaim any and all liability to any person in respect of anything or the consequences of anything done or omitted to be done in reliance upon the whole or any part of this document. Minister’s Foreword Building vibrant and sustainable communities is a complex, multi-layered process but at its heart is one critically important component – the views of the community itself. There is growing recognition both in Australia and internationally that engaging the community in both plan making and development assessment processes results in better planning outcomes. That is why one of the key principles of planFIRST – the biggest reforms to the NSW planning system in more than two decades – is greater community engagement in the planning and development system. -

Government Gazette of the STATE of NEW SOUTH WALES Number 174 Wednesday, 28 November 2007 Published Under Authority by Government Advertising
8657 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 174 Wednesday, 28 November 2007 Published under authority by Government Advertising SPECIAL SUPPLEMENT EXOTIC DISEASES OF ANIMALS ACT 1991 ORDER – Section 15 Declaration of Restricted Area – Special Restricted Area (Purple) – Tamworth to Camden I, IAN JAMES ROTH, Deputy Chief Veterinary Offi cer, with the powers the Minister has delegated to me under section 67 of the Exotic Diseases of Animals Act 1991 (‘the Act’) and pursuant to section 15 of the Act and being of the opinion that the area specifi ed in Schedule 1 may be or become infected with the exotic disease Equine infl uenza hereby: 1. revoke the order declared under section 15 of the Act titled “Declaration of Restricted Area – Special Restricted Area (Purple) Tamworth to Camden” dated 2 November 2007 and any order revived as a result of this revocation; 2. declare the area specifi ed in Schedule 1 to be a restricted area, to be known as the “Special Restricted Area (Purple) – Greater Purple”; and 3. declare the areas specifi ed in Schedule 2 to be a restricted area, to be known as “Special Restricted Area (Purple) – Tamworth to Camden” as shown on the map in Schedule 2 below; and 4. declare that the classes of animals, animal products, fodder, fi ttings or vehicles to which this order applies are those described in Schedule 3. SCHEDULE 1 Special Restricted Area (Purple) – Greater Purple 1. That area comprising the parishes of NSW and suburbs of Sydney listed in the table below except the area described as follows: The area -

2015/16 Annual Review
ANNUAL REVIEW 15/16 PMS > CMYK > REVERSED > PROVIDING REGIONAL WATER AUTHORITIES WITH INDEPENDENT, EXPERT ADVICE, TECHNICAL SUPPORT, SHARED INDUSTRY KNOWLEDGE, IMPROVED EFFICIENCIES AND LONG TERM PLANNING. CHAIR’S REVIEW In 2015/16 the Water Directorate made notable is the eleventh Executive Committee member advances in the face of change and challenges. to reach this milestone. Very special mention The year commenced with NSW Office of Water goes to Wayne Beatty, Water and Sewerage advising its new name of DPI Water and that Strategic Manager at Orange City Council, for it will focus on water planning and policy in his dedicated support of the Water Directorate. urban and rural areas, and will also oversee At the March Executive Committee meeting I government funded water infrastructure presented Wayne with a 15-year medallion and programs and develop more information on thanked him and Orange City Council for his water for the community. Final structural input and advised that Wayne is only the fourth arrangements and the impact on urban water Executive Committee member to achieve this branch within DPI Water are still being resolved. significant milestone. Highest number of members yet Important links with the wider water industry I was extremely pleased when the 98th council In these interesting times we place great value joined the Water Directorate: our highest level of on our relationships with Local Government membership in 18 years. We appreciate this show NSW, IPWEA, AWA, WSAA and WIOA. of support from our member councils throughout On a lighter note, at the WIOA Conference in 2015/16. Representation is 96% of the102 NSW Newcastle, Nambucca Shire Council was judged local water utilities - but ironically this milestone to have the best tasting NSW water in 2016.