Phylogeny and Zoogeography of <I>Laemonema</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Teleostei: Gadiformes: Moridae), Antimora Microlepis and Physiculus Japonicus, from the Western North Pacific
Species Diversity, 2007, 12, 17–27 Pelagic Juveniles of Two Morids (Teleostei: Gadiformes: Moridae), Antimora microlepis and Physiculus japonicus, from the Western North Pacific Makoto Okamoto1, Naoshi Sato2, Takashi Asahida2 and Yoshiro Watanabe3 1 Tohoku National Fisheries Research Institute, 3-27-5 Shinhama-cho, Shiogama, Miyagi, 985-0001 Japan E-mail: [email protected] 2 School of Fisheries Sciences, Kitasato University, 160-4 Okirai, Sanriku-cho, Ofunato, Iwate, 022-0101 Japan 3 Ocean Research Institute, University of Tokyo, Minamidai, Nakano-ku, Tokyo, 164-8639 Japan (Received 1 July 2006; Accepted 20 November 2006) Pelagic juveniles of two morids, Antimora microlepis Bean, 1890 and Physiculus japonicus Hilgendorf, 1879, were collected by midwater trawl (0–20 m depth) from transition waters between the Oyashio and Kuroshio fronts of the western North Pacific in May, 1989. Juveniles of A. microlepis (13 specimens, 30.2–54.3 mm standard length, SL) characteristically have an elongated body, posteriorly positioned anus, 24–25 precaudal vertebrae, chin with a barbel, no ventral luminous organ, elongated pelvic fin rays, and a non-protruding snout. Juveniles of P. japonicus (six specimens, 20.4–39.0 mm SL) characteristically have an elongated body, chin with a barbel, ventral lu- minous organ anterior to the anus, elongated pelvic fin rays, a pointed cau- dal fin, 9–10 dorsal fin rays, 66–70 second dorsal fin rays, 68–72 anal fin rays, six pelvic fin rays, 16ϩ41–42 vertebrae, and ca. 130 longitudinal scales. Key Words: Teleostei, Gadiformes, Moridae, Antimora microlepis, Physicu- lus japonicus, pelagic juvenile. Introduction The family Moridae currently comprises 18 genera and about 110 species, which occur widely from coastal to pelagic waters over the entire world except in the Arctic Ocean (Paulin 1989a; Okamura 1995; Eschmeyer 1998). -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Ices/Nafo Wgdec Report 2015
ICES/NAFO WGDEC REPORT 2015 ICES ADVISORY COMMITTEE ICES CM 2015/ACOM:27 Report of the ICES/NAFO Joint Working Group on Deep-water Ecology (WGDEC) 16–20 February 2015 Horta, Azores, Portugal International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2015. Report of the ICES/NAFO Joint Working Group on Deep-water Ecology (WGDEC), 16–20 February 2015, Horta, Azores, Portugal. ICES CM 2015/ACOM:27. 113 pp. For permission to reproduce material from this publication, please apply to the Gen- eral Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2015 International Council for the Exploration of the Sea ICES/NAFO WGDEC REPORT 2015 | i Contents Executive summary ................................................................................................................ 4 Opening of the meeting ........................................................................................................ 5 1 Adoption of the agenda ................................................................................................ 6 2 Provide all available new information on distribution of VMEs in the North Atlantic with a view to advising on any new closures to bottom fisheries or revision of existing closures to bottom fisheries (NEAFC standing request). In addition, provide new information on location of habitats sensitive to particular fishing activities (i.e. vulnerable marine ecosystems, VMEs) within EU waters (EC request) ................................... 8 2.1 Introduction ........................................................................................................... 8 2.2 Areas within the NEAFC regulatory area ........................................................ -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -
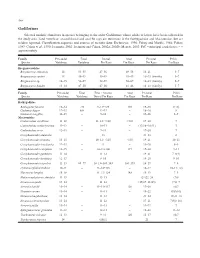
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Temporal and Spatial Management Tools for Marine Ecosystems: Case Studies from Northern Brazil and Northeastern United States
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses October 2019 TEMPORAL AND SPATIAL MANAGEMENT TOOLS FOR MARINE ECOSYSTEMS: CASE STUDIES FROM NORTHERN BRAZIL AND NORTHEASTERN UNITED STATES Beatriz dos Santos Dias University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation dos Santos Dias, Beatriz, "TEMPORAL AND SPATIAL MANAGEMENT TOOLS FOR MARINE ECOSYSTEMS: CASE STUDIES FROM NORTHERN BRAZIL AND NORTHEASTERN UNITED STATES" (2019). Doctoral Dissertations. 1714. https://doi.org/10.7275/15232062 https://scholarworks.umass.edu/dissertations_2/1714 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. TEMPORAL AND SPATIAL MANAGEMENT TOOLS FOR MARINE ECOSYSTEMS: CASE STUDIES FROM NORTHERN BRAZIL AND NORTHEASTERN UNITED STATES A Dissertation Presented by BEATRIZ DOS SANTOS DIAS Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment Of the requirement for the degree of DOCTOR OF PHILOSOPHY September 2019 Department of Environmental Conservation Wildlife, Fish, and Conservation Biology © Copyright by Beatriz dos Santos Dias 2019 All Rights Reserved TEMPORAL AND SPATIAL MANAGEMENT TOOLS FOR MARINE ECOSYSTEMS: CASE STUDIES FROM NORTHERN BRAZIL AND NORTHEASTERN UNITED STATES A Dissertation Presented By BEATRIZ DOS SANTOS DIAS Approved as to style and content by: ____________________________________________ Adrian Jordaan, Chair ____________________________________________ John T. Finn, Member ____________________________________________ Michael G. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2006 Rosenstiel School of Marine and Atmospheric Science, University of Miami. This manuscript is available at http://www.rsmas.miami.edu/bms and may be cited as: Reed, J. K., Weaver, D. C., & Pomponi, S. A. (2006). Habitat and fauna of deep-water Lophelia pertusa coral reefs off the southeastern US: Blake Plateau, Straits of Florida, and Gulf of Mexico. Bulletin of Marine Science, 78(2), 343-375. BULLETIN OF MARINE SCIENCE, 78(2): 343–375, 2006 CORAL REEF PAPER Habitat anD Fauna of Deep-Water LOPHELIA PERTUSA Coral Reefs off the Southeastern U.S.: BlaKE Plateau, Straits of FloriDA, anD Gulf of MEXico John K. Reed, Doug C. Weaver, and Shirley A. Pomponi Abstract Expeditions from 1999 to 2004 for biomedical research explored various deep-sea coral ecosystems (DSCE) off the southeastern U.S. (Blake Plateau, Straits of Florida, and eastern Gulf of Mexico). Habitat and benthos were documented from 57 dives with human occupied submersibles and three with a remotely operated vehicle (ROV), and resulted in ~100 hrs of videotapes, 259 in situ digital images, 621 muse- um specimens, and > 400 microbial isolates. These were the first dives to document the habitat, benthic fauna, and fish diversity of some of these poorly known deep- water reefs. Fifty-eight fish species and 142 benthic invertebrate taxa were identi- fied. High-definition topographic SEABEAM maps and echosounder profiles were also produced. Sites included in this report range from South Carolina on the Blake Plateau to the southwestern Florida slope: 1) Stetson Lophelia reefs along the east- ern Blake Plateau off South Carolina; 2) Savannah Lophelia lithoherms along the western Blake Plateau off Georgia; 3) east Florida Lophelia reefs, 4) Miami Terrace escarpment in the Straits of Florida; 5) Pourtalès Terrace off the Florida Keys; and 6) west Florida Lophelia lithoherms off the southwestern Florida shelf in the Gulf of Mexico. -

Determining the Diet of New Zealand King Shag Using DNA Metabarcoding
Determining the diet of New Zealand king shag using DNA metabarcoding New Zealand King Shag, (Leucocarbo carunculatus) on Blumine Island, Marlborough Sounds, New Zealand in 2016 (Wikipedia commons). Aimee van der Reis & Andrew Jeffs Report Prepared For: Department of Conservation, Conservation Services Programme, Project BCBC2019-05. DOC MarineDRAFT Science Advisors Graeme Taylor and Dr Karen Middlemiss. November 2020 Reports from Auckland UniServices Limited should only be used for the purposes for which they were commissioned. If it is proposed to use a report prepared by Auckland UniServices Limited for a different purpose or in a different context from that intended at the time of commissioning the work, then UniServices should be consulted to verify whether the report is being correctly interpreted. In particular it is requested that, where quoted, conclusions given in Auckland UniServices reports should be stated in full. INTRODUCTION The New Zealand king shag (Leucocarbo carunculatus) is an endemic seabird that is classed as nationally endangered (Miskelly et al., 2008). The population is confined to a small number of colonies located around the coastal margins of the outer Marlborough Sounds (South Island, New Zealand); with surveys suggesting the population is currently stable (~800 individuals surveyed in 2020; Aquaculture New Zealand, 2020; Schuckard et al., 2015). Monitoring the colonies has become a priority and research is being conducted to better understand their population dynamics and basic ecology to improve the management of the population, particularly in relation to human activities such as fishing, aquaculture and land use (Fisher & Boren, 2012). The diet of the New Zealand king shag is strongly linked to the waters surrounding their colonies and it has been suggested that anthropogenic activities, such as marine farm structures, may displace foraging habitat that could affect the population of New Zealand king shag (Fisher & Boren, 2012). -

MOR 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) MORIDAE Moras Body Relatively Elongate, Tapering To
click for previous page MOR 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) MORIDAE Moras Body relatively elongate, tapering to a narrow caudal peduncle. Mouth terminal or inferior; teeth few or lacking on head of vomer (roof of mouth). No spines in fins; 2 or 3 dorsal fins and 1 or 2 anal fins; pelvic fins thoracic, never very close together; caudal fin always separate from dorsal and anal fins. Small cycloid scales on body and head. Anterior paired projections of swimbladder attached to a membranous area at the rear of the cranium. Colour: variable, black or grey to light brown or pink; some species may have iridescent areas. Fishes of the continental slope and abyssal depths. Locally abundant in some habitats but not commercial in the area. Taken in bottom trawls. Lepidion Physiculus Tripterophycis - 2 - FAO Sheets MORIDAE Fishing Area 51 SIMILAR FAMILIES OCCURRING IN THE AREA: Macrouridae: no caudal fin. Ophidiidae and Bythitidae: ventral fins close Macrouridae together, dorsal,caudal and anal fins joined in most. Ateleopodidae: no scales; no free caudal fin; a single dorsal fin far forward on the body. Ophidiidae Ateleopodidae pelvic fins Moridae Ophidiidae Underside of head KEY TO GENERA OCCURRING IN THE AREA: 1a. Mouth inferior, beneath a prominent, flattened, pointed, bony snout (Fig.1) ................................ Antimora 1b. Mouth terminal to slightly inferior; snout nor- mal Antimora Fig.1 2a. One or more small, dark, scaleless patches (covering a light organ) on the belly (Fig.2) patch covering light organ pelvic fin anus Fig.2 - 3 - FAO Sheets MORIDAE Fishing Area 51 3a.