Extraoral Anatomy in CBCT - Michael M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Head and Neck
DEFINITION OF ANATOMIC SITES WITHIN THE HEAD AND NECK adapted from the Summary Staging Guide 1977 published by the SEER Program, and the AJCC Cancer Staging Manual Fifth Edition published by the American Joint Committee on Cancer Staging. Note: Not all sites in the lip, oral cavity, pharynx and salivary glands are listed below. All sites to which a Summary Stage scheme applies are listed at the begining of the scheme. ORAL CAVITY AND ORAL PHARYNX (in ICD-O-3 sequence) The oral cavity extends from the skin-vermilion junction of the lips to the junction of the hard and soft palate above and to the line of circumvallate papillae below. The oral pharynx (oropharynx) is that portion of the continuity of the pharynx extending from the plane of the inferior surface of the soft palate to the plane of the superior surface of the hyoid bone (or floor of the vallecula) and includes the base of tongue, inferior surface of the soft palate and the uvula, the anterior and posterior tonsillar pillars, the glossotonsillar sulci, the pharyngeal tonsils, and the lateral and posterior walls. The oral cavity and oral pharynx are divided into the following specific areas: LIPS (C00._; vermilion surface, mucosal lip, labial mucosa) upper and lower, form the upper and lower anterior wall of the oral cavity. They consist of an exposed surface of modified epider- mis beginning at the junction of the vermilion border with the skin and including only the vermilion surface or that portion of the lip that comes into contact with the opposing lip. -

A Ally Long Epiglottis: a Case Report
Case Report Unusually long epiglottis: A case report Azhar A Siddiqui 1*, A G Shroff 2 1Professor, Department of Anatomy, Indian Institute of Medical Science and Research, Warudi, Tq. Badnapur, Dist. Jalna 2Dean, MGM Medical College, Aurangabad, Maharashtra, INDIA. Email : [email protected] Abstract The Epiglottis is thin leaf shaped cartilage of larynx attached to other cartilages of larynx, hyoid bone and tongue either directly or by mucosal folds. It is usually longer and higher in children than adults. Usually the epiglottis is not seen on oral examination, as it lies below the level of tongue. However rarely, it may be seen in chil dren if it is unusually long labeled as Visible Epiglottis, High Raising Epiglottis or High Arched Epiglottis, etc... A rare case report of Unusually Long Epiglottis is presented in an adult female, detected accidently during routine oral examination for c ommon cold. The patient was not having any complaints because of this condition. Literature states that this condition is rarely seen in children but very rare in adults. If asymptomatic it should be left alone with assurance to the patient and relatives. It may be treated only if creating obstruction to airway. Keywords: High-rising epiglottis, Long Epiglottis, Visible Epiglottis. *Address for Correspondence: Dr. Azhar A. Siddiqui, Professor, Flat No. 1, Saidham Apartment, Jaisingpura, Near University Gate, Aurangabad – 431001, Maharashtra, INDIA. Email: [email protected] Received Date: 25/04/2015 Revised Date: 04/0 5/2015 Accepted Date: 06/05/2015 Development: The epiglottis devel ops from fusion of Access this article online ventral ends of fourth arch with caudal part of hypobronchial eminence. -

Human Anatomy As Related to Tumor Formation Book Four
SEER Program Self Instructional Manual for Cancer Registrars Human Anatomy as Related to Tumor Formation Book Four Second Edition U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutesof Health SEER PROGRAM SELF-INSTRUCTIONAL MANUAL FOR CANCER REGISTRARS Book 4 - Human Anatomy as Related to Tumor Formation Second Edition Prepared by: SEER Program Cancer Statistics Branch National Cancer Institute Editor in Chief: Evelyn M. Shambaugh, M.A., CTR Cancer Statistics Branch National Cancer Institute Assisted by Self-Instructional Manual Committee: Dr. Robert F. Ryan, Emeritus Professor of Surgery Tulane University School of Medicine New Orleans, Louisiana Mildred A. Weiss Los Angeles, California Mary A. Kruse Bethesda, Maryland Jean Cicero, ART, CTR Health Data Systems Professional Services Riverdale, Maryland Pat Kenny Medical Illustrator for Division of Research Services National Institutes of Health CONTENTS BOOK 4: HUMAN ANATOMY AS RELATED TO TUMOR FORMATION Page Section A--Objectives and Content of Book 4 ............................... 1 Section B--Terms Used to Indicate Body Location and Position .................. 5 Section C--The Integumentary System ..................................... 19 Section D--The Lymphatic System ....................................... 51 Section E--The Cardiovascular System ..................................... 97 Section F--The Respiratory System ....................................... 129 Section G--The Digestive System ......................................... 163 Section -

II. DIGESTIV SYSTEM TESTS General Data 1. CS the Organ Represent: A
II. DIGESTIV SYSTEM TESTS General data 1. CS The organ represent: a) a structure made up by three layers b) a hollow element c) a part of the body built by complex of tissues integrated to realize the common functions d) a parenchymatous formation located in abdominal cavity e) a formation constituted by epithelium, vessels and nerves 2. CS The visceral apparatus is considered: a) The organs of different systems with diverse structure involved in performing some functions. b) the organs of neck region c) the organs located in the lesser pelvis d) the organs realized protective function e) the organs located at the border between thoracic and abdominal cavities 3. CS The primary gut is developed from: a) ectoderm b) mesoderm c) endoderm d) dermatome e) myotome 4. CS From which embryonic layer is developed the primary intestine : a) entoderm b) ectoderm c) sclerotome d) mesoderm e) splanhnopleura 5. CM The Viscera represents: a) the organs localized in abdominal cavity b) the systems of organs realized the connection of the body and external environment c) the organs and system of organs located in body’s cavities which realized the metabolic functions to sustain the life d) the complex of organs from abdominal and pelvic cavities e) the complex of organs from thoracic cavity 6. CM According by structure the organs are divided in: a) serous b) parenchymatous c) glandular d) epithelial e) hollow 7. CM Name two functions of the organic stroma: a) secretory b) trophic c) hematopoietic d) metabolic e) sustaining 8. CM The hollow organs distinguish the following layers: a) mucous b) submucous c) muscular d) membranous e) serous 9. -

Ear Pain in Patients with Oropharynx Carcinoma: Karlt.Beer Peter Vock How MRI Contributes to the Explanation Richard H
Eur Radiol (2004) 14:2206–2211 DOI 10.1007/s00330-004-2340-2 HEAD AND NECK Harriet C. Thoeny Ear pain in patients with oropharynx carcinoma: KarlT.Beer Peter Vock how MRI contributes to the explanation Richard H. Greiner of a prognostic and predictive symptom Received: 22 October 2003 Abstract Reflex otalgia is a predic- glossus muscle, stylopharyngeus Revised: 11 March 2004 tive and prognostic parameter for lo- muscle, hyoglossus muscle and pre- Accepted: 5 April 2004 cal control in patients with orophar- epiglottic space. No difference was Published online: 1 May 2004 ynx carcinoma. Can a morphologic found for the muscles of mastication, © Springer-Verlag 2004 correlate of this important symptom levator and tensor veli palatini mus- be detected by MRI? Thirty-six pa- cles, styloglossus muscle, genioglos- tients were prospectively evaluated sus muscle, intrinsic muscles of the by MRI before radical radiotherapy. tongue, digastric muscles, mucosal Sixteen patients had reflex otalgia; surface of the lateral and posterior 20 did not. The oropharynx and adja- pharyngeal wall, uvula, valleculae, cent regions were analyzed. Alter- parapharyngeal space and larynx. An ation was defined as effacement of alteration of structures innervated by H. C. Thoeny (✉) · P. Vock anatomical structures, signal alter- the glossopharyngeal nerve was vi- Department of Diagnostic Radiology, ation or enhancement after contrast sualized on MRI significantly more Inselspital, χ2 University of Bern, medium administration. The -test often when reflex otalgia was pres- Freiburgstrasse 10, 3010 Bern, Switzerland was used to compare categorical pa- ent. Involvement of structures inner- e-mail: [email protected], rameters. In patients with reflex vated by other cranial nerves did not [email protected] otalgia, alteration of the following show the same association with ear Tel.: +41-31-6322939 structures innervated by the glosso- pain. -

Volume 1: the Upper Extremity
Volume 1: The Upper Extremity 1.1 The Shoulder 01.00 - 38.20 (37.20) 1.1.1 Introduction to shoulder section 0.01.00 0.01.28 0.28 1.1.2 Bones, joints, and ligaments 1 Clavicle, scapula 0.01.29 0.05.40 4.11 1.1.3 Bones, joints, and ligaments 2 Movements of scapula 0.05.41 0.06.37 0.56 1.1.4 Bones, joints, and ligaments 3 Proximal humerus 0.06.38 0.08.19 1.41 Shoulder joint (glenohumeral joint) Movements of shoulder joint 1.1.5 Review of bones, joints, and ligaments 0.08.20 0.09.41 1.21 1.1.6 Introduction to muscles 0.09.42 0.10.03 0.21 1.1.7 Muscles 1 Long tendons of biceps, triceps 0.10.04 0.13.52 3.48 Rotator cuff muscles Subscapularis Supraspinatus Infraspinatus Teres minor Teres major Coracobrachialis 1.1.8 Muscles 2 Serratus anterior 0.13.53 0.17.49 3.56 Levator scapulae Rhomboid minor and major Trapezius Pectoralis minor Subclavius, omohyoid 1.1.9 Muscles 3 Pectoralis major 0.17.50 0.20.35 2.45 Latissimus dorsi Deltoid 1.1.10 Review of muscles 0.20.36 0.21.51 1.15 1.1.11 Vessels and nerves: key structures First rib 0.22.09 0.24.38 2.29 Cervical vertebrae Scalene muscles 1.1.12 Blood vessels 1 Veins of the shoulder region 0.24.39 0.27.47 3.08 1.1.13 Blood vessels 2 Arteries of the shoulder region 0.27.48 0.30.22 2.34 1.1.14 Nerves The brachial plexus and its branches 0.30.23 0.35.55 5.32 1.1.15 Review of vessels and nerves 0.35.56 0.38.20 2.24 1.2. -

Functional Anatomy of the Soft Palate Applied to Wind Playing
Review Functional Anatomy of the Soft Palate Applied to Wind Playing Alison Evans, MMus, Bronwen Ackermann, PhD, and Tim Driscoll, PhD Wind players must be able to sustain high intraoral pressures in dition occurs because of a structural deformity, such as with order to play their instruments. Prolonged exposure to these high cleft palate. It is also associated with some other speech dis- pressures may lead to the performance-related disorder velopharyn- orders. VPI occurs when the soft palate fails to completely geal insufficiency (VPI). This disorder occurs when the soft palate fails to completely close the air passage between the oral and nasal close the oronasal cavity while attempting to blow air through cavities in the upper respiratory cavity during blowing tasks, this clo- the mouth, resulting in air escaping from the nose.5 Without sure being necessary for optimum performance on a wind instru- a tight air seal, the air passes into the nasal cavity and can ment. VPI is potentially career threatening. Improving music teach- then escape out the nose. This has a disastrous effect on wind ers’ and students’ knowledge of the mechanism of velopharyngeal playing, as the power behind the wind musicians’ sound closure may assist in avoiding potentially catastrophic performance- related disorders arising from dysfunction of the soft palate. In the relies on enough controlled expired air through the mouth. functional anatomy of the soft palate as applied to wind playing, Understandably, this disorder may potentially end the musi- seven muscles of the soft palate involved in the velopharyngeal clo- cian’s career.6 sure mechanism are reviewed. -

Appendix B: Muscles of the Speech Production Mechanism
Appendix B: Muscles of the Speech Production Mechanism I. MUSCLES OF RESPIRATION A. MUSCLES OF INHALATION (muscles that enlarge the thoracic cavity) 1. Diaphragm Attachments: The diaphragm originates in a number of places: the lower tip of the sternum; the first 3 or 4 lumbar vertebrae and the lower borders and inner surfaces of the cartilages of ribs 7 - 12. All fibers insert into a central tendon (aponeurosis of the diaphragm). Function: Contraction of the diaphragm draws the central tendon down and forward, which enlarges the thoracic cavity vertically. It can also elevate to some extent the lower ribs. The diaphragm separates the thoracic and the abdominal cavities. 2. External Intercostals Attachments: The external intercostals run from the lip on the lower border of each rib inferiorly and medially to the upper border of the rib immediately below. Function: These muscles may have several functions. They serve to strengthen the thoracic wall so that it doesn't bulge between the ribs. They provide a checking action to counteract relaxation pressure. Because of the direction of attachment of their fibers, the external intercostals can raise the thoracic cage for inhalation. 3. Pectoralis Major Attachments: This muscle attaches on the anterior surface of the medial half of the clavicle, the sternum and costal cartilages 1-6 or 7. All fibers come together and insert at the greater tubercle of the humerus. Function: Pectoralis major is primarily an abductor of the arm. It can, however, serve as a supplemental (or compensatory) muscle of inhalation, raising the rib cage and sternum. (In other words, breathing by raising and lowering the arms!) It is mentioned here chiefly because it is encountered in the dissection. -

Anatomy and Physiology of the Velopharyngeal Mechanism
Anatomy and Physiology of the Velopharyngeal Mechanism Jamie L. Perry, Ph.D.1 ABSTRACT Understanding the normal anatomy and physiology of the velopharyngeal mechanism is the first step in providing appropriate diagnosis and treatment for children born with cleft lip and palate. The velopharyngeal mechanism consists of a muscular valve that extends from the posterior surface of the hard palate (roof of mouth) to the posterior pharyngeal wall and includes the velum (soft palate), lateral pharyngeal walls (sides of the throat), and the posterior pharyngeal wall (back wall of the throat). The function of the velopharyngeal mechanism is to create a tight seal between the velum and pharyngeal walls to separate the oral and nasal cavities for various purposes, including speech. Velopharyngeal closure is accomplished through the contraction of several velopharyngeal muscles including the levator veli palatini, musculus uvulae, superior pharyngeal con- strictor, palatopharyngeus, palatoglossus, and salpingopharyngeus. The tensor veli palatini is thought to be responsible for eustachian tube function. KEYWORDS: Anatomy, physiology, velopharyngeal muscles, cleft palate anatomy Downloaded by: SASLHA. Copyrighted material. Learning Outcomes: As a result of this activity, the reader will be able to (1) list the major muscles of the velopharyngeal mechanism and discuss their functions; (2) list the sensory and motor innervation patterns for the muscles of the velopharyngeal mechanism; and (3) discuss the variations in velopharyngeal anatomy found in an unrepaired cleft palate. Understanding the normal anatomy and and treatment for children born with cleft lip physiology of the velopharyngeal mechanism is and palate. Most of the diagnostic and therapy the first step in providing appropriate diagnosis approaches are based on a strong foundation of 1Department of Communication Sciences and Disorders, Guest Editor, Ann W. -
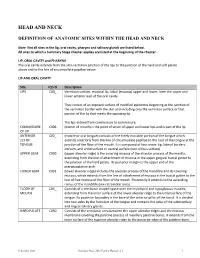
Head and Neck: Summary Stage 2018 Coding Manual V2.0
HEAD AND NECK DEFINITION OF ANATOMIC SITES WITHIN THE HEAD AND NECK Note: Not all sites in the lip, oral cavity, pharynx and salivary glands are listed below. All sites to which a Summary Stage chapter applies are listed at the beginning of the chapter. LIP, ORAL CAVITY and PHARYNX The oral cavity extends from the skin-vermilion junction of the lips to the junction of the hard and soft palate above and to the line of circumvallate papillae below. LIP AND ORAL CAVITY Site ICD-O Description LIPS C00_ Vermilion surface, mucosal lip, labial (mucosa) upper and lower, form the upper and lower anterior wall of the oral cavity. They consist of an exposed surface of modified epidermis beginning at the junction of the vermilion border with the skin and including only the vermilion surface or that portion of the lip that meets the opposing lip. The lips extend from commissure to commissure. COMMISSURE C006 (corner of mouth) is the point of union of upper and lower lips and is part of the lip OF LIP ANTERIOR C02_ (mobile or oral tongue) consists of the freely movable portion of the tongue which 2/3 OF extends anteriorly from the line of circumvallate papillae to the root of the tongue at the TONGUE junction of the floor of the mouth. It is composed of four areas: tip, lateral borders, dorsum, and undersurface or ventral surface (non-villous surface). UPPER GUM C030 (upper alveolar ridge) is the covering mucosa of the alveolar process of the maxilla, extending from the line of attachment of mucosa in the upper gingival buccal gutter to the junction of the hard palate. -

Mvdr. Natália Hvizdošová, Phd. Mudr. Zuzana Kováčová
MVDr. Natália Hvizdošová, PhD. MUDr. Zuzana Kováčová ABDOMEN Borders outer: xiphoid process, costal arch, Th12 iliac crest, anterior superior iliac spine (ASIS), inguinal lig., mons pubis internal: diaphragm (on the right side extends to the 4th intercostal space, on the left side extends to the 5th intercostal space) plane through terminal line Abdominal regions superior - epigastrium (regions: epigastric, hypochondriac left and right) middle - mesogastrium (regions: umbilical, lateral left and right) inferior - hypogastrium (regions: pubic, inguinal left and right) ABDOMINAL WALL Orientation lines xiphisternal line – Th8 subcostal line – L3 bispinal line (transtubercular) – L5 Clinically important lines transpyloric line – L1 (pylorus, duodenal bulb, fundus of gallbladder, superior mesenteric a., cisterna chyli, hilum of kidney, lower border of spinal cord) transumbilical line – L4 Bones Lumbar vertebrae (5): body vertebral arch – lamina of arch, pedicle of arch, superior and inferior vertebral notch – intervertebral foramen vertebral foramen spinous process superior articular process – mammillary process inferior articular process costal process – accessory process Sacrum base of sacrum – promontory, superior articular process lateral part – wing, auricular surface, sacral tuberosity pelvic surface – transverse lines (ridges), anterior sacral foramina dorsal surface – median, intermediate, lateral sacral crest, posterior sacral foramina, sacral horn, sacral canal, sacral hiatus apex of the sacrum Coccyx coccygeal horn Layers of the abdominal wall 1. SKIN 2. SUBCUTANEOUS TISSUE + SUPERFICIAL FASCIAS + SUPRAFASCIAL STRUCTURES Superficial fascias: Camper´s fascia (fatty layer) – downward becomes dartos m. Scarpa´s fascia (membranous layer) – downward becomes superficial perineal fascia of Colles´) dartos m. + Colles´ fascia = tunica dartos Suprafascial structures: Arteries and veins: cutaneous brr. of posterior intercostal a. and v., and musculophrenic a. -

Comparative Myology and Evolution of Marsupials and Other Vertebrates, with Notes on Complexity, Bauplan, and “Scala Naturae”
RVC OPEN ACCESS REPOSITORY – COPYRIGHT NOTICE This is the peer reviewed version of: Diogo, R., Bello-Hellegouarch, G., Kohlsdorf, T., Esteve-Altava, B. and Molnar, J. L. (2016), Comparative Myology and Evolution of Marsupials and Other Vertebrates, With Notes on Complexity, Bauplan, and “Scala Naturae”. Anat. Rec., 299: 1224–1255. doi:10.1002/ar.23390 which has been published in final form at http://dx.doi.org/10.1002/ar.23390. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. The full details of the published version of the article are as follows: TITLE: Comparative Myology and Evolution of Marsupials and Other Vertebrates, With Notes on Complexity, Bauplan, and "Scala Naturae" AUTHORS: Diogo, R., Bello-Hellegouarch, G., Kohlsdorf, T., Esteve-Altava, B. and Molnar, J. L. JOURNAL TITLE: Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology PUBLISHER: Wiley PUBLICATION DATE: September 2016 DOI: 10.1002/ar.23390 Comparative myology and evolution of marsupials and other vertebrates, with notes on complexity, Bauplan, and ‘scala naturae’ Rui Diogo1, Gaelle Bello-Hellegouarch2, Tiana Kohlsdorf2, Borja Esteve-Altava1,3, Julia L. Molnar1 1 Department of Anatomy, Howard University College of Medicine, Numa Adams Building, 520 W St. NW, Washington, DC 20059, US. 2 Department of Biology, FFCLRP. University of São Paulo. Avenida Bandeirantes, 3900. Bairro Monte 7 Alegre. Ribeirão Preto, SP. Brazil. 3 Structure & Motion Laboratory, Department of Comparative Biomedical Sciences, Royal Veterinary College, Hawkshead Lane, Hatfield, Hertfordshire AL9 7TA, United Kingdom. Corresponding author: Rui Diogo. Department of Anatomy, Howard University College of Medicine, Numa Adams Building, 520 W St.