Diptera: Cecidomyiidae): Morphological and Behavioural Evidence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Willows of Interior Alaska
1 Willows of Interior Alaska Dominique M. Collet US Fish and Wildlife Service 2004 2 Willows of Interior Alaska Acknowledgements The development of this willow guide has been made possible thanks to funding from the U.S. Fish and Wildlife Service- Yukon Flats National Wildlife Refuge - order 70181-12-M692. Funding for printing was made available through a collaborative partnership of Natural Resources, U.S. Army Alaska, Department of Defense; Pacific North- west Research Station, U.S. Forest Service, Department of Agriculture; National Park Service, and Fairbanks Fish and Wildlife Field Office, U.S. Fish and Wildlife Service, Department of the Interior; and Bonanza Creek Long Term Ecological Research Program, University of Alaska Fairbanks. The data for the distribution maps were provided by George Argus, Al Batten, Garry Davies, Rob deVelice, and Carolyn Parker. Carol Griswold, George Argus, Les Viereck and Delia Person provided much improvement to the manuscript by their careful editing and suggestions. I want to thank Delia Person, of the Yukon Flats National Wildlife Refuge, for initiating and following through with the development and printing of this guide. Most of all, I am especially grateful to Pamela Houston whose support made the writing of this guide possible. Any errors or omissions are solely the responsibility of the author. Disclaimer This publication is designed to provide accurate information on willows from interior Alaska. If expert knowledge is required, services of an experienced botanist should be sought. Contents -

Dipterists Forum
BULLETIN OF THE Dipterists Forum Bulletin No. 76 Autumn 2013 Affiliated to the British Entomological and Natural History Society Bulletin No. 76 Autumn 2013 ISSN 1358-5029 Editorial panel Bulletin Editor Darwyn Sumner Assistant Editor Judy Webb Dipterists Forum Officers Chairman Martin Drake Vice Chairman Stuart Ball Secretary John Kramer Meetings Treasurer Howard Bentley Please use the Booking Form included in this Bulletin or downloaded from our Membership Sec. John Showers website Field Meetings Sec. Roger Morris Field Meetings Indoor Meetings Sec. Duncan Sivell Roger Morris 7 Vine Street, Stamford, Lincolnshire PE9 1QE Publicity Officer Erica McAlister [email protected] Conservation Officer Rob Wolton Workshops & Indoor Meetings Organiser Duncan Sivell Ordinary Members Natural History Museum, Cromwell Road, London, SW7 5BD [email protected] Chris Spilling, Malcolm Smart, Mick Parker Nathan Medd, John Ismay, vacancy Bulletin contributions Unelected Members Please refer to guide notes in this Bulletin for details of how to contribute and send your material to both of the following: Dipterists Digest Editor Peter Chandler Dipterists Bulletin Editor Darwyn Sumner Secretary 122, Link Road, Anstey, Charnwood, Leicestershire LE7 7BX. John Kramer Tel. 0116 212 5075 31 Ash Tree Road, Oadby, Leicester, Leicestershire, LE2 5TE. [email protected] [email protected] Assistant Editor Treasurer Judy Webb Howard Bentley 2 Dorchester Court, Blenheim Road, Kidlington, Oxon. OX5 2JT. 37, Biddenden Close, Bearsted, Maidstone, Kent. ME15 8JP Tel. 01865 377487 Tel. 01622 739452 [email protected] [email protected] Conservation Dipterists Digest contributions Robert Wolton Locks Park Farm, Hatherleigh, Oakhampton, Devon EX20 3LZ Dipterists Digest Editor Tel. -

IOBC/WPRS Working Group “Integrated Plant Protection in Fruit
IOBC/WPRS Working Group “Integrated Plant Protection in Fruit Crops” Subgroup “Soft Fruits” Proceedings of Workshop on Integrated Soft Fruit Production East Malling (United Kingdom) 24-27 September 2007 Editors Ch. Linder & J.V. Cross IOBC/WPRS Bulletin Bulletin OILB/SROP Vol. 39, 2008 The content of the contributions is in the responsibility of the authors The IOBC/WPRS Bulletin is published by the International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palearctic Regional Section (IOBC/WPRS) Le Bulletin OILB/SROP est publié par l‘Organisation Internationale de Lutte Biologique et Intégrée contre les Animaux et les Plantes Nuisibles, section Regionale Ouest Paléarctique (OILB/SROP) Copyright: IOBC/WPRS 2008 The Publication Commission of the IOBC/WPRS: Horst Bathon Luc Tirry Julius Kuehn Institute (JKI), Federal University of Gent Research Centre for Cultivated Plants Laboratory of Agrozoology Institute for Biological Control Department of Crop Protection Heinrichstr. 243 Coupure Links 653 D-64287 Darmstadt (Germany) B-9000 Gent (Belgium) Tel +49 6151 407-225, Fax +49 6151 407-290 Tel +32-9-2646152, Fax +32-9-2646239 e-mail: [email protected] e-mail: [email protected] Address General Secretariat: Dr. Philippe C. Nicot INRA – Unité de Pathologie Végétale Domaine St Maurice - B.P. 94 F-84143 Montfavet Cedex (France) ISBN 978-92-9067-213-5 http://www.iobc-wprs.org Integrated Plant Protection in Soft Fruits IOBC/wprs Bulletin 39, 2008 Contents Development of semiochemical attractants, lures and traps for raspberry beetle, Byturus tomentosus at SCRI; from fundamental chemical ecology to testing IPM tools with growers. -

Role of the Predator, Aphidoletes Aphidimyza (Rondani) (Diptera: Cecidomyiidae), in the Management of the Apple Aphid, Aphis Pomi Degeer (Homoptera: Aphididae)
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1977 Role of the predator, Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae), in the management of the apple aphid, Aphis pomi DeGeer (Homoptera: Aphididae). Roger Gilbert Adams University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Adams, Roger Gilbert, "Role of the predator, Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae), in the management of the apple aphid, Aphis pomi DeGeer (Homoptera: Aphididae)." (1977). Doctoral Dissertations 1896 - February 2014. 5616. https://scholarworks.umass.edu/dissertations_1/5616 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. ROLE OF THE PREDATOR, APHIDOLETES APHIDIMYZA (RQNDANl) (DIPTERA CECIDOMYIIDAE), IN THE MANAGEMENT OF THE APPLE APHID, APHIS POMI DEGEER (HOMOPTERA: APHIDIDAE). A Dissertation Presented By Roger Gilbert Adams, Jr* Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY September 1977 Department of Entomology i ROLE CF THE PREDATOR, APHIDOLETES APHIDIMYZA (RONDANl) (DIPTERA: • CECIDOMmDAE), IN THE MANAGEMENT OP THE APPLE APHID, APHIS POMI DEGEER (HOMOPTERA: APHIDIDAE), A Dissertation Presented By Roger Gilbert Adams, Jr* Approved as to style and content toy: A -'J / At ft l (Dr* Ronald J* Prokopy), Chairperson of Committee /\ ,, , . • ^ // ( i e,-/ A Ut // ^ U 1* 'l i. /i'\ ,1, (Dr* Richard A* Damon, Jr*), Member ii ACKNOWLEDGEMENTS I wish to express my deep appreciation to my advisor, Dr. -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
_____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ I MUNIS ENTOMOLOGY & ZOOLOGY Ankara / Turkey II _____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ Scope: Munis Entomology & Zoology publishes a wide variety of papers on all aspects of Entomology and Zoology from all of the world, including mainly studies on systematics, taxonomy, nomenclature, fauna, biogeography, biodiversity, ecology, morphology, behavior, conservation, paleobiology and other aspects are appropriate topics for papers submitted to Munis Entomology & Zoology. Submission of Manuscripts: Works published or under consideration elsewhere (including on the internet) will not be accepted. At first submission, one double spaced hard copy (text and tables) with figures (may not be original) must be sent to the Editors, Dr. Hüseyin Özdikmen for publication in MEZ. All manuscripts should be submitted as Word file or PDF file in an e-mail attachment. If electronic submission is not possible due to limitations of electronic space at the sending or receiving ends, unavailability of e-mail, etc., we will accept “hard” versions, in triplicate, accompanied by an electronic version stored in a floppy disk, a CD-ROM. Review Process: When submitting manuscripts, all authors provides the name, of at least three qualified experts (they also provide their address, subject fields and e-mails). Then, the editors send to experts to review the papers. The review process should normally be completed within 45-60 days. After reviewing papers by reviwers: Rejected papers are discarded. For accepted papers, authors are asked to modify their papers according to suggestions of the reviewers and editors. Final versions of manuscripts and figures are needed in a digital format. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Phylogeny and Host Association in Platygaster Latreille, 1809 (Hymenoptera, Platygastridae)
Phylogeny and host association in Platygaster Latreille, 1809 (Hymenoptera, Platygastridae) Peter Neerup Buhl Buhl, P.N.: Phylogeny and host association in Platygaster Latreille, 1809 (Hy menoptera, Platygastridae). Ent. Meddr 69: 113-122. Copenhagen, Denmark 2001. ISSN 0013-8851. An examination of the known midge host/midge plant host associations for species of Platygasterparasitoid wasps seems to indicate a number of natural par asitoid species groups restricted to specific plant families. Midge hosts seem less indicative for platygastrid relationships, but several exceptions from this rule exist. The possible reasons for this are discussed. It is also shown that species of Platy gaster with known host associations generally prefer midges on plant families which are not the families generally prefered by the midges. Furthermore, a com parison of the known midge host/midge plant host associations for the genera of the "Platygaster-cluster" and the "Synopeas-cluster" shows great differences in the general preferences of the clusters. P.N. Buhl, Troldh0jvej 3, DK-3310 0lsted, Denmark. E-mail: [email protected] Introduction The phylogeny of the very large platygastrid genus Platygaster, tiny parasitoids on gall midges (Diptera, Cecidomyiidae), is mostly unresolved. The great problems which meet the investigator are primarily - as in all platygastrids - the few external characters avail able in a phylogenetic analysis. A further obstacle in the revisionary work is that many species are known only from short dated original descriptions (unknown or unrevised type material). Aspects of the biology (midge host or host plant of midge) are, however, known for about half the described species, so perhaps this could enlighten aspects of the parasitoid taxonomy- as was successfully done e.g. -
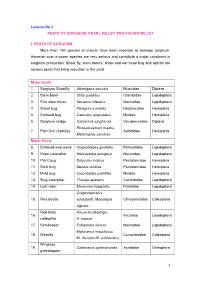
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

Checklist of Lithuanian Diptera
Acta Zoologica Lituanica. 2000. Volumen 10. Numerus 1 3 ISSN 1392-1657 CHECKLIST OF LITHUANIAN DIPTERA Saulius PAKALNIÐKIS1, Jolanta RIMÐAITË1, Rasa SPRANGAUSKAITË-BERNOTIENË1, Rasa BUTAUTAITË2, Sigitas PODËNAS2 1 Institute of Ecology, Akademijos 2, 2600 Vilnius, Lithuania 2 Department of Zoology, Vilnius University, M.K. Èiurlionio 21/27, 2009 Vilnius, Lithuania Abstract. The list of 2283 Lithuanian Diptera species of 78 families is based on 224 literary sources. Key words: Diptera, Acroceridae, Agromyzidae, Anisopodidae, Anthomyiidae, Anthomyzidae, Asilidae, Athericidae, Bibionidae, Bombyliidae, Calliphoridae, Cecidomyiidae, Ceratopogonidae, Chama- emyiidae, Chaoboridae, Chironomidae, Chloropidae, Coelopidae, Conopidae, Culicidae, Cylindroto- midae, Diastatidae, Ditomyiidae, Dixidae, Dolichopodidae, Drosophilidae, Dryomyzidae, Empididae, Ephydridae, Fanniidae, Gasterophilidae, Helcomyzidae, Heleomyzidae, Hippoboscidae, Hybotidae, Hypodermatidae, Lauxaniidae, Limoniidae, Lonchaeidae, Lonchopteridae, Macroceridae, Megamerinidae, Micropezidae, Microphoridae, Muscidae, Mycetophilidae, Neottiophilidae, Odiniidae, Oestridae, Opo- myzidae, Otitidae, Pallopteridae, Pediciidae, Phoridae, Pipunculidae, Platypezidae, Psilidae, Psychod- idae, Ptychopteridae, Rhagionidae, Sarcophagidae, Scathophagidae, Scatopsidae, Scenopinidae, Sciaridae, Sciomyzidae, Sepsidae, Simuliidae, Sphaeroceridae, Stratiomyidae, Syrphidae, Tabanidae, Tachinidae, Tephritidae, Therevidae, Tipulidae, Trichoceridae, Xylomyidae, Xylophagidae, checklist, Lithuania INTRODUCTION -

Klicken, Um Den Anhang Zu Öffnen
Gredleria- VOL. 1 / 2001 Titelbild 2001 Posthornschnecke (Planorbarius corneus L.) / Zeichnung: Alma Horne Volume 1 Impressum Volume Direktion und Redaktion / Direzione e redazione 1 © Copyright 2001 by Naturmuseum Südtirol Museo Scienze Naturali Alto Adige Museum Natöra Südtirol Bindergasse/Via Bottai 1 – I-39100 Bozen/Bolzano (Italien/Italia) Tel. +39/0471/412960 – Fax 0471/412979 homepage: www.naturmuseum.it e-mail: [email protected] Redaktionskomitee / Comitato di Redazione Dr. Klaus Hellrigl (Brixen/Bressanone), Dr. Peter Ortner (Bozen/Bolzano), Dr. Gerhard Tarmann (Innsbruck), Dr. Leo Unterholzner (Lana, BZ), Dr. Vito Zingerle (Bozen/Bolzano) Schriftleiter und Koordinator / Redattore e coordinatore Dr. Klaus Hellrigl (Brixen/Bressanone) Verantwortlicher Leiter / Direttore responsabile Dr. Vito Zingerle (Bozen/Bolzano) Graphik / grafica Dr. Peter Schreiner (München) Zitiertitel Gredleriana, Veröff. Nat. Mus. Südtirol (Acta biol. ), 1 (2001): ISSN 1593 -5205 Issued 15.12.2001 Druck / stampa Gredleriana Fotolito Varesco – Auer / Ora (BZ) Gredleriana 2001 l 2001 tirol Die Veröffentlichungsreihe »Gredleriana« des Naturmuseum Südtirol (Bozen) ist ein Forum für naturwissenschaftliche Forschung in und über Südtirol. Geplant ist die Volume Herausgabe von zwei Wissenschaftsreihen: A) Biologische Reihe (Acta Biologica) mit den Bereichen Zoologie, Botanik und Ökologie und B) Erdwissenschaftliche Reihe (Acta Geo lo gica) mit Geologie, Mineralogie und Paläontologie. Diese Reihen können jährlich ge mein sam oder in alternierender Folge erscheinen, je nach Ver- fügbarkeit entsprechender Beiträge. Als Publikationssprache der einzelnen Beiträge ist Deutsch oder Italienisch vorge- 1 Naturmuseum Südtiro sehen und allenfalls auch Englisch. Die einzelnen Originalartikel erscheinen jeweils Museum Natöra Süd Museum Natöra in der eingereichten Sprache der Autoren und sollen mit kurzen Zusammenfassun- gen in Englisch, Italienisch und Deutsch ausgestattet sein. -

Gall Midges (Diptera: Cecidomyiidae) New to the Danish Fauna
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/309155026 Gall midges (Diptera: Cecidomyiidae) new to the Danish fauna Article · October 2016 CITATIONS READS 0 779 4 authors: Simon Haarder Hans Henrik Bruun University of Copenhagen University of Copenhagen 31 PUBLICATIONS 74 CITATIONS 120 PUBLICATIONS 2,624 CITATIONS SEE PROFILE SEE PROFILE Keith Harris Marcela Skuhravá 32 PUBLICATIONS 274 CITATIONS 88 PUBLICATIONS 559 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Historical Danish plant biodiversity View project Deep history or current environment? Determinants of landscape-level grassland plant diversity. View project All content following this page was uploaded by Hans Henrik Bruun on 14 October 2016. The user has requested enhancement of the downloaded file. Ent. Tidskr. 137 (2016) New gall midges from Denmark Gall midges (Diptera: Cecidomyiidae) new to the Danish fauna SIMON HAARDER, HANS HENRIK BRUUN, KEITH M. HARRIS & MARCELA SKUHRAVÁ Haarder, S., Bruun, H.H., Harris, K.M. & Skuhravá, M.: Gall midges (Diptera: Cecidomy- iidae) new to the Danish fauna. [Nya gallmyggor (Diptera: Cecidomyiidae) för den danska faunan.] – Entomologisk Tidskrift 137(3): 79-98. Uppsala, Sweden 2016. ISSN 0013-886x. First records of twenty-three gall midge species in Denmark are reported: Asphondylia ervi Rübsaamen, Contarinia acetosellae Rübsaamen, C. viburnorum Kieffer, Dasineura astragalorum (Kieffer), D. fructum (Rübsaamen), D. harrisoni (Bagnall), D. lotharingi- ae (Kieffer), D. papaveris (Winnertz), D. saxifragae (Kieffer), D. traili (Kieffer), Her- bomyia robusta Möhn, Jaapiella chelidonii Fedotova, Lasioptera arundinis Schiner, L. calamagrostidis Rübsaamen, Mayetiola festucae Ertel, M. -
NDP 41 Hessian
NDP 41 V1- National Diagnostic Protocol for Mayetiola destructor National Diagnostic Protocol Mayetiola destructor Hessian Fly NDP 41 V1 NDP 41 V1 - National Diagnostic Protocol for Mayetiola destructor © Commonwealth of Australia Ownership of intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth). Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from http://creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from https://creativecommons.org/licenses/by/3.0/au/legalcode. This publication (and any material sourced from it) should be attributed as: Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee on Plant Health Diagnostics) Authors Severtson, D, Szito, A.; Reviewers Nicholas, A, Kehoe, M. ISBN 978-0-6481143-3-8 CC BY 3.0. Cataloguing data Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee