Revision of the Family <I>Dipterocarpaceae</I> In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Further Breeding Records for Birds (Aves) in Angola
Durban Natural Science Museum Novitates 36 ANGOLAN BIRD BREEDING RECORDS 1 FURTHER BREEDING RECORDS FOR BIRDS (AVES) IN ANGOLA W. RicHARD J. DeAn1*, URSULA FRAnKe2, GRAnT JOSePH1, FRANCIScO M. GOnÇALVeS3, MicHAeL S.L. MiLLS4,1, SUZAnne J. MiLTOn1, ARA MOnADJeM5 & H. DieTeR OScHADLeUS6 1DST/NRF Centre of Excellence at the Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa *Author for correspondence: [email protected] 2Tal 34, 80331 Munich, Germany 3ISCED, Department of Natural Sciences, Rua: Sarmento Rodrigues, P.O. Box 230, Lubango, Angola 4A.P. Leventis Ornithological Research Institute, University of Jos, P.O. Box 13404, Jos, Plateau State, Nigeria 5Department of Biological Sciences, University of Swaziland, Private Bag 4, Kwaluseni, Swaziland 6Animal Demography Unit, Department of Zoology, University of Cape Town, Rondebosch 7701, South Africa ean, W.R.J., Franke, U., Joseph, G., Gonçalves, F.M., Mills, M.S.L., Milton, S.J., Monadjem, A. D& Oschadleus, H.D. 2013. Further breeding records for birds (Aves) in Angola. Durban Natural Science Museum Novitates 36: 1-10. Some details of records of nests, eggs and nestlings of 167 (possibly 168) species in the bird collection at Lubango, Angola are given. This includes 23 species for which there were no Angolan breeding records at all, and one possibly new breeding species (Slaty Egret). The data also confirm the breeding of another 20 species strongly suspected of breeding in Angola, but that lacked egg or nestling records. KEYWORDS: Angola, birds, museum collections, breeding. INTRODUcTiOn SYSTeMATIC LiST One of the gaps in our knowledge of the natural history of birds in Taxonomy and order follows Gill & Donsker (2014). -
Total Solar Eclipse of 2002 December 4
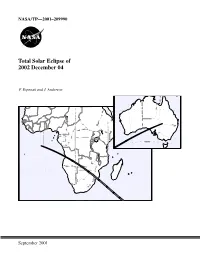
NASA/TP—2001–209990 Total Solar Eclipse of 2002 December 04 F. Espenak and J. Anderson Central Lat,Lng = -28.0 132.0 P Factor = 0.46 Semi W,H = 0.35 0.28 Offset X,Y = 0.00-0.00 1999 Oct 26 10:40:42 AM High Res World Data [WPD1] WorldMap v2.00, F. Espenak Orthographic Projection Scale = 8.00 mm/° = 1:13915000 Central Lat,Lng = -10.0 26.0 P Factor = 0.31 Semi W,H = 0.70 0.50 Offset X,Y = 0.00-0.00 1999 Oct 26 10:17:57 AM September 2001 The NASA STI Program Office … in Profile Since its founding, NASA has been dedicated to • CONFERENCE PUBLICATION. Collected the advancement of aeronautics and space papers from scientific and technical science. The NASA Scientific and Technical conferences, symposia, seminars, or other Information (STI) Program Office plays a key meetings sponsored or cosponsored by NASA. part in helping NASA maintain this important role. • SPECIAL PUBLICATION. Scientific, techni- cal, or historical information from NASA The NASA STI Program Office is operated by programs, projects, and mission, often con- Langley Research Center, the lead center for cerned with subjects having substantial public NASA’s scientific and technical information. The interest. NASA STI Program Office provides access to the NASA STI Database, the largest collection of • TECHNICAL TRANSLATION. aeronautical and space science STI in the world. English-language translations of foreign scien- The Program Office is also NASA’s institutional tific and technical material pertinent to NASA’s mechanism for disseminating the results of its mission. -

Pakaraimaea Dipterocarpacea
The Ectomycorrhizal Fungal Community in a Neotropical Forest Dominated by the Endemic Dipterocarp Pakaraimaea dipterocarpacea Matthew E. Smith1*, Terry W. Henkel2, Jessie K. Uehling2, Alexander K. Fremier3, H. David Clarke4, Rytas Vilgalys5 1 Department of Plant Pathology, University of Florida, Gainesville, Florida, United States of America, 2 Department of Biological Sciences, Humboldt State University, Arcata, California, United States of America, 3 Department of Fish and Wildlife Resources, University of Idaho, Moscow, Idaho, United States of America, 4 Department of Biology, University of North Carolina Asheville, Asheville, North Carolina, United States of America, 5 Department of Biology, Duke University, Durham, North Carolina, United States of America Abstract Ectomycorrhizal (ECM) plants and fungi can be diverse and abundant in certain tropical ecosystems. For example, the primarily paleotropical ECM plant family Dipterocarpaceae is one of the most speciose and ecologically important tree families in Southeast Asia. Pakaraimaea dipterocarpacea is one of two species of dipterocarp known from the Neotropics, and is also the only known member of the monotypic Dipterocarpaceae subfamily Pakaraimoideae. This Guiana Shield endemic is only known from the sandstone highlands of Guyana and Venezuela. Despite its unique phylogenetic position and unusual geographical distribution, the ECM fungal associations of P. dipterocarpacea are understudied throughout the tree’s range. In December 2010 we sampled ECM fungi on roots of P. dipterocarpacea and the co-occurring ECM tree Dicymbe jenmanii (Fabaceae subfamily Caesalpinioideae) in the Upper Mazaruni River Basin of Guyana. Based on ITS rDNA sequencing we documented 52 ECM species from 11 independent fungal lineages. Due to the phylogenetic distance between the two host tree species, we hypothesized that P. -

Guerra Em Angola As Heranças Da Luta De Libertação E a Guerra Civil
ACADEMIA MILITAR DIRECÇÃO DE ENSINO Mestrado em Ciências Militares – Especialidade de Cavalaria Trabalho de Investigação Aplicada GUERRA EM ANGOLA AS HERANÇAS DA LUTA DE LIBERTAÇÃO E A GUERRA CIVIL Elaborado por: Asp Tir Cav Feliciano Paulo Agostinho (RA) Orientador: TCor Cav Francisco Amado Rodrigues Lisboa, Setembro de 2011 ACADEMIA MILITAR DIRECÇÃO DE ENSINO Mestrado em Ciências Militares – Especialidade de Cavalaria Trabalho de Investigação Aplicada GUERRA EM ANGOLA AS HERANÇAS DA LUTA DE LIBERTAÇÃO E A GUERRA CIVIL Elaborado por: Asp Cav Feliciano Paulo Agostinho (RA) Orientador: TCor Cav Francisco Amado Rodrigues Lisboa, Setembro de 2011 DEDICATÓRIA À minha família, em especial à minha mãe, esposa e filha. Muito obrigado pelo amor, carinho e apoio prestados. GUERRA EM ANGOLA - AS HERANÇAS DA LUTA DE LIBERTAÇÃO E A GUERRA CIVIL iii AGRADECIMENTOS Manifesto o contentamento por todo o apoio prestado à realização deste trabalho de investigação. O meu muito obrigado: Ao Comandante da Academia Militar, Tenente-General Paiva Monteiro, por permitir que esta investigação fosse possível; Ao Tenente-Coronel de Cavalaria Francisco Amado Rodrigues, meu orientador, por todo o esclarecimento e orientação concedidos; À direcção do curso de cavalaria, na pessoa do Tenente-Coronel de Cavalaria Henrique Mateus, pelo apoio, disponibilidade e partilha de conhecimentos; À Chancelaria de Angola em Portugal, pelo apoio material e financeiro prestado durante a sua realização; Ao Tenente-Coronel de Cavalaria Paulo Ramos, na ajuda da escolha do tema e na elaboração da questão principal; Ao Tenente-Coronel de Infantaria António Marracho, pelo apoio inicial, na definição e estrutura do trabalho; Ao Tenente de Artilharia Agostinho Silva, pela compreensão, apoio e dedicação; À Biblioteca Nacional do Palácio Gouveia e ao Museu da República e Resistência, por toda a atenção prestada durante as pesquisas; À Biblioteca da Academia Militar, em especial à Sra. -

Closterium Thailandicum N
Tropical palynofloras from Middle Miocene Chiang Muan basin, Phayao, Thailand Wickanet Songtham1, Benjavun Ratanasthien2 and Dallas C. Mildenhall3 1 Bureau of Geological Survey, Department of Geological Resources, Rama VI Road, Ratchathevee, Bangkok 10400, Thailand 2 Department of Geological Sciences, Faculty of Science, Chiang Mai University, Chiang Mai 50200 Thailand 3 Institute of Geological and Nuclear Sciences, Lower Hutt, P.O. Box 30-368 New Zealand Á¦¼´µ °¡¦¦Å¤oÁ ¦°µÂ°o Án ¸¥¤nª ´®ª¡³Á¥µ´ ª·Á« ¦¦¦¤1, ÁȪ¦¦ ¦´Á¸¥¦2 ¨³ Dallas C. Mildenhall3 1 ε´¦¸ª¥µ· ¦¤¦´¡¥µ¦¦¸ ¡¦³¦µ¤¸É 6 ¦µÁª¸ ¦»Á¡¤®µ¦ 10400 2 £µª·µ¦ª¸ ·¥µ ³ª·¥µ«µ¦r ¤®µª·¥µ¨´¥Á¸¥Ä®¤n ´®ª´Á¥Ä®¤¸ n 50200 3 Institute of Geological and Nuclear Sciences, Lower Hutt, P.O. Box 30-368 New Zealand ABSTRACT Sporomorphs from sediments of Middle Miocene Chiang Muan basin include abundant Crassoretitriletes vanraadshoovenii, Actinastrum bansaense n. sp., and Closterium thailandicum n. sp., and common Dipterocarpaceae, Lagerstroemia, Ilexpollenites, Botryococcus, Myrtaceidites, and Combretum with rare forms of Florschuetzia, Homonoia, Calophyllum, Striatriletes susannae, and Mimosoideae. There are abundant Laevigatosporites haardtii fern spores in some horizons with various forms of as yet unidentified tricolporate and tricolpate pollen. The sporomorphs, representing tropical palynofloras derived from tropical monsoon forests, accumulated mainly in lacustrine depositional environments. Origins and distributions of the families Dipterocarpaceae and Myrtaceae are discussed and new criteria elucidating their paleophytogeographic histories in relation to northern Thailand are proposed. ´¥n° Á¦¼´µ °¨³°°Á¦ °¦r ¨³µ®¦nµ¥µ³°¤´¥Å¤Ã°¸°¨µµÂ°nÁ¸¥ ¤nªÃÁnoª¥°¦r ° Crassoretitriletes vanraadshoovenii µ®¦nµ¥Êεº Actinastrum bansaense n. -

2854 ISS Monograph 130.Indd
FFROMROM SSOLDIERSOLDIERS TTOO CCITIZENSITIZENS THE SOCIAL, ECONOMIC AND POLITICAL REINTEGRATION OF UNITA EX-COMBATANTS J GOMES PORTO, IMOGEN PARSONS AND CHRIS ALDEN ISS MONOGRAPH SERIES • No 130, MARCH 2007 CONTENTS ACKNOWLEDGEMENTS iii ABOUT THE AUTHORS v LIST OF ACRONYMS vi INTRODUCTION viii CHAPTER ONE 1 Angola’s Central Highlands: Provincial Characterisation and Fieldwork Review CHAPTER TWO 39 Unita’s Demobilised Soldiers: Portrait of the post-Luena target group CHAPTER THREE 53 The Economic, Social and Political Dimensions of Reintegration: Findings CHAPTER FOUR 79 Surveying for Trends: Correlation of Findings CHAPTER FIVE 109 From Soldiers to Citizens: Concluding Thoughts ENDNOTES 127 BIBLIOGRAPHY 139 ANNEX 145 Survey Questionnaire iii ACKNOWLEDGMENTS The research and publication of this monograph were made possible by the generous funding of the Swedish International Development Cooperation Agency (SIDA), the Swiss Federal Department of Foreign Affairs, and the Norwegian Institute of International Affairs (NUPI), through the African Security Analysis Programme at the ISS. The project “From Soldiers to Citizens: A study of the social, economic and political reintegration of UNITA ex-combatants in post-war Angola” was developed jointly by the African Security Analysis Programme at ISS, the London School of Economics and Political Science (LSE), and the Norwegian Institute for International Affairs (NUPI). In addition, the project established a number of partnerships with Angolan non-governmental organisations (NGOs), including Development -

2.3 Angola Road Network
2.3 Angola Road Network Distance Matrix Travel Time Matrix Road Security Weighbridges and Axle Load Limits For more information on government contact details, please see the following link: 4.1 Government Contact List. Page 1 Page 2 Distance Matrix Uige – River Nzadi bridge 18 m-long and 4 m-wide near the locality of Kitela, north of Songo municipality destroyed during civil war and currently under rehabilitation (news 7/10/2016). Road Details Luanda The Government/MPLA is committed to build 1,100 km of roads in addition to 2,834 km of roads built in 2016 and planned rehabilitation of 7,083 km of roads in addition to 10,219 km rehabilitated in 2016. The Government goals will have also the support from the credit line of the R. of China which will benefit inter-municipality links in Luanda, Uige, Malanje, Cuanza Norte, Cuanza Sul, Benguela, Huambo and Bié provinces. For more information please vitsit the Website of the Ministry of Construction. Zaire Luvo bridge reopened to trucks as of 15/11/2017, this bridge links the municipality of Mbanza Congo with RDC and was closed for 30 days after rehabilitation. Three of the 60 km between MCongo/Luvo require repairs as of 17/11/2017. For more information please visit the Website of Agencia Angola Press. Works of rehabilitation on the road nr, 120 between Mbanza Congo (province Zaire) and the locality of Lukunga (province of Uige) of a distance of 111 km are 60% completed as of 29/9/2017. For more information please visit the Website of Agencia Angola Press. -

Phylogeny of the Tropical Tree Family Dipterocarpaceae Based on Nucleotide Sequences of the Chloroplast Rbcl Gene1
American Journal of Botany 86(8): 1182±1190. 1999. PHYLOGENY OF THE TROPICAL TREE FAMILY DIPTEROCARPACEAE BASED ON NUCLEOTIDE SEQUENCES OF THE CHLOROPLAST RBCL GENE1 S. DAYANANDAN,2,6 PETER S. ASHTON,3 SCOTT M. WILLIAMS,4 AND RICHARD B. PRIMACK2 2Biology Department, Boston University, Boston, Massachusetts 02215; 3Harvard University Herbaria, 22 Divinity Avenue, Cambridge, Massachusetts 02138; and 4Division of Biomedical Sciences, Meharry Medical College, 1005 D. B. Todd, Jr. Boulevard, Nashville, Tennessee 37208 The Dipterocarpaceae, well-known trees of the Asian rain forests, have been variously assigned to Malvales and Theales. The family, if the Monotoideae of Africa (30 species) and South America and the Pakaraimoideae of South America (one species) are included, comprises over 500 species. Despite the high diversity and ecological dominance of the Dipterocar- paceae, phylogenetic relationships within the family as well as between dipterocarps and other angiosperm families remain poorly de®ned. We conducted parsimony analyses on rbcL sequences from 35 species to reconstruct the phylogeny of the Dipterocarpaceae. The consensus tree resulting from these analyses shows that the members of Dipterocarpaceae, including Monotes and Pakaraimaea, form a monophyletic group closely related to the family Sarcolaenaceae and are allied to Malvales. The present generic and higher taxon circumscriptions of Dipterocarpaceae are mostly in agreement with this molecular phylogeny with the exception of the genus Hopea, which forms a clade with Shorea sections Anthoshorea and Doona. Phylogenetic placement of Dipterocarpus and Dryobalanops remains unresolved. Further studies involving repre- sentative taxa from Cistaceae, Elaeocarpaceae, Hopea, Shorea, Dipterocarpus, and Dryobalanops will be necessary for a comprehensive understanding of the phylogeny and generic limits of the Dipterocarpaceae. -

Karyomorphology and Its Evolution in Dipterocarpaceae (Malvales)
© 2020 The Japan Mendel Society Cytologia 85(2): 141–149 Karyomorphology and Its Evolution in Dipterocarpaceae (Malvales) Kazuo Oginuma1*, Shawn Y. K. Lum2 and Hiroshi Tobe3 1 The Community Center for the Advancement of Education and Research at the University of Kochi, 5–15 Eikokuji-cho, Kochi 780–8515, Japan 2 Asian School of the Environment, Nanyang Technological University, Singapore 639798 3 Department of Botany, Graduate School of Science, Kyoto University, Kyoto 606–8502, Japan Received January 16, 2020; accepted February 9, 2020 Summary Previous chromosome information is restricted to Dipterocarpoideae, one of the two subfamilies of Dipterocarpaceae, and no chromosome information is available for another subfamily Monotoideae. Here we present the first karyomorphology of Marquesia macroura (2n=22) (Monotoideae), as well as of four species (2n=22) of four genera in tribe Dipterocarpeae and five species (2n=14) of tribe Shoreae in Dipterocarpoideae. Comparisons within Dipterocarpaceae and with Sarcolaenaceae (2n=22) sister to Dipetrocarpaceae in the light of phylogenetic relationships show that the basic chromosome number x=11 is plesiomorphic and x=7 apomor- phic in Dipterocapaceae. Based on available information, tribe Shoreae (x=7) has a uniform karyotype where all chromosomes have a centromere at median position, while the rest of the family (x=11) have a diverse karyotype in terms of the frequency of chromosomes with a centromere at median, submedian and subterminal position. We discussed the meaning of lability of karyotype in chromosome evolution. Keywords Basic chromosome number, Chromosome evolution, Dipterocarpaceae, Karyomorphology. Dipterocarpaceae (Malvales) are a family of 16 gen- x=10, and five genera Dryobalanops, Hopea, Neobala- era and 680 species distributed in tropical regions of nocarpus, Parashorea and Shorea of tribe Shoreae all the Old World, especially in the rain forests of Malesia have x=7. -

Inventário Florestal Nacional, Guia De Campo Para Recolha De Dados
Monitorização e Avaliação de Recursos Florestais Nacionais de Angola Inventário Florestal Nacional Guia de campo para recolha de dados . NFMA Working Paper No 41/P– Rome, Luanda 2009 Monitorização e Avaliação de Recursos Florestais Nacionais As florestas são essenciais para o bem-estar da humanidade. Constitui as fundações para a vida sobre a terra através de funções ecológicas, a regulação do clima e recursos hídricos e servem como habitat para plantas e animais. As florestas também fornecem uma vasta gama de bens essenciais, tais como madeira, comida, forragem, medicamentos e também, oportunidades para lazer, renovação espiritual e outros serviços. Hoje em dia, as florestas sofrem pressões devido ao aumento de procura de produtos e serviços com base na terra, o que resulta frequentemente na degradação ou transformação da floresta em formas insustentáveis de utilização da terra. Quando as florestas são perdidas ou severamente degradadas. A sua capacidade de funcionar como reguladores do ambiente também se perde. O resultado é o aumento de perigo de inundações e erosão, a redução na fertilidade do solo e o desaparecimento de plantas e animais. Como resultado, o fornecimento sustentável de bens e serviços das florestas é posto em perigo. Como resposta do aumento de procura de informações fiáveis sobre os recursos de florestas e árvores tanto ao nível nacional como Internacional l, a FAO iniciou uma actividade para dar apoio à monitorização e avaliação de recursos florestais nationais (MANF). O apoio à MANF inclui uma abordagem harmonizada da MANF, a gestão de informação, sistemas de notificação de dados e o apoio à análise do impacto das políticas no processo nacional de tomada de decisão. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M. -

Nazrin Full Phd Thesis (150246576
Maintenance and conservation of Dipterocarp diversity in tropical forests _______________________________________________ Mohammad Nazrin B Abdul Malik A thesis submitted in partial fulfilment of the degree of Doctor of Philosophy Faculty of Science Department of Animal and Plant Sciences November 2019 1 i Thesis abstract Many theories and hypotheses have been developed to explain the maintenance of diversity in plant communities, particularly in hyperdiverse tropical forests. Maintenance of the composition and diversity of tropical forests is vital, especially species of high commercial value. I focus on the high value dipterocarp timber species of Malaysia and Borneo as these have been extensive logged owing to increased demands from global timber trade. In this thesis, I explore the drivers of diversity of this group, as well as the determinants of global abundance, conservation and timber value. The most widely supported hypothesis for explaining tropical diversity is the Janzen Connell hypothesis. I experimentally tested the key elements of this, namely density and distance dependence, in two dipterocarp species. The results showed that different species exhibited different density and distance dependence effects. To further test the strength of this hypothesis, I conducted a meta-analysis combining multiple studies across tropical and temperate study sites, and with many species tested. It revealed significant support for the Janzen- Connell predictions in terms of distance and density dependence. Using a phylogenetic comparative approach, I highlight how environmental adaptation affects dipterocarp distribution, and the relationships of plant traits with ecological factors and conservation status. This analysis showed that environmental and ecological factors are related to plant traits and highlights the need for dipterocarp conservation priorities.