Population Dynamics of the Surf Clams Donax Hanleyanus and Mesodesma Mactroides from Open-Atlantic Beaches Off Argentina
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project
High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project AEA Technology, Environment Contract: W/35/00632/00/00 For: The Department of Trade and Industry New & Renewable Energy Programme Report issued 30 August 2002 (Version with minor corrections 16 September 2002) Keith Hiscock, Harvey Tyler-Walters and Hugh Jones Reference: Hiscock, K., Tyler-Walters, H. & Jones, H. 2002. High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Report from the Marine Biological Association to The Department of Trade and Industry New & Renewable Energy Programme. (AEA Technology, Environment Contract: W/35/00632/00/00.) Correspondence: Dr. K. Hiscock, The Laboratory, Citadel Hill, Plymouth, PL1 2PB. [email protected] High level environmental screening study for offshore wind farm developments – marine habitats and species ii High level environmental screening study for offshore wind farm developments – marine habitats and species Title: High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Contract Report: W/35/00632/00/00. Client: Department of Trade and Industry (New & Renewable Energy Programme) Contract management: AEA Technology, Environment. Date of contract issue: 22/07/2002 Level of report issue: Final Confidentiality: Distribution at discretion of DTI before Consultation report published then no restriction. Distribution: Two copies and electronic file to DTI (Mr S. Payne, Offshore Renewables Planning). One copy to MBA library. Prepared by: Dr. K. Hiscock, Dr. H. Tyler-Walters & Hugh Jones Authorization: Project Director: Dr. Keith Hiscock Date: Signature: MBA Director: Prof. S. Hawkins Date: Signature: This report can be referred to as follows: Hiscock, K., Tyler-Walters, H. -

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Geoducks—A Compendium
34, NUMBER 1 VOLUME JOURNAL OF SHELLFISH RESEARCH APRIL 2015 JOURNAL OF SHELLFISH RESEARCH Vol. 34, No. 1 APRIL 2015 JOURNAL OF SHELLFISH RESEARCH CONTENTS VOLUME 34, NUMBER 1 APRIL 2015 Geoducks — A compendium ...................................................................... 1 Brent Vadopalas and Jonathan P. Davis .......................................................................................... 3 Paul E. Gribben and Kevin G. Heasman Developing fisheries and aquaculture industries for Panopea zelandica in New Zealand ............................... 5 Ignacio Leyva-Valencia, Pedro Cruz-Hernandez, Sergio T. Alvarez-Castaneda,~ Delia I. Rojas-Posadas, Miguel M. Correa-Ramırez, Brent Vadopalas and Daniel B. Lluch-Cota Phylogeny and phylogeography of the geoduck Panopea (Bivalvia: Hiatellidae) ..................................... 11 J. Jesus Bautista-Romero, Sergio Scarry Gonzalez-Pel aez, Enrique Morales-Bojorquez, Jose Angel Hidalgo-de-la-Toba and Daniel Bernardo Lluch-Cota Sinusoidal function modeling applied to age validation of geoducks Panopea generosa and Panopea globosa ................. 21 Brent Vadopalas, Jonathan P. Davis and Carolyn S. Friedman Maturation, spawning, and fecundity of the farmed Pacific geoduck Panopea generosa in Puget Sound, Washington ............ 31 Bianca Arney, Wenshan Liu, Ian Forster, R. Scott McKinley and Christopher M. Pearce Temperature and food-ration optimization in the hatchery culture of juveniles of the Pacific geoduck Panopea generosa ......... 39 Alejandra Ferreira-Arrieta, Zaul Garcıa-Esquivel, Marco A. Gonzalez-G omez and Enrique Valenzuela-Espinoza Growth, survival, and feeding rates for the geoduck Panopea globosa during larval development ......................... 55 Sandra Tapia-Morales, Zaul Garcıa-Esquivel, Brent Vadopalas and Jonathan Davis Growth and burrowing rates of juvenile geoducks Panopea generosa and Panopea globosa under laboratory conditions .......... 63 Fabiola G. Arcos-Ortega, Santiago J. Sanchez Leon–Hing, Carmen Rodriguez-Jaramillo, Mario A. -

Improving the NEFSC Clam Survey for Atlantic Surfclams and Ocean Quahogs
Northeast Fisheries Science Center Reference Document 19-06 Improving the NEFSC Clam Survey for Atlantic Surfclams and Ocean Quahogs by Larry Jacobson and Daniel Hennen May 2019 Northeast Fisheries Science Center Reference Document 19-06 Improving the NEFSC Clam Survey for Atlantic Surfclams and Ocean Quahogs by Larry Jacobson and Daniel Hennen NOAA Fisheries, Northeast Fisheries Science Center, 166 Water Street, Woods Hole, MA 02543 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Northeast Fisheries Science Center Woods Hole, Massachusetts May 2019 Northeast Fisheries Science Center Reference Documents This series is a secondary scientific seriesdesigned to assure the long-term documentation and to enable the timely transmission of research results by Center and/or non-Center researchers, where such results bear upon the research mission of the Center (see the outside back cover for the mission statement). These documents receive internal scientific review, and most receive copy editing. The National Marine Fisheries Service does not endorse any proprietary material, process, or product mentioned in these documents. If you do not have Internet access, you may obtain a paper copy of a document by contacting the senior Center author of the desired document. Refer to the title page of the document for the senior Center author’s name and mailing address. If there is no Center author, or if there is corporate (i.e., non-individualized) authorship, then contact the Center’s Woods Hole Labora- tory Library (166 Water St., Woods Hole, MA 02543-1026). Information Quality Act Compliance: In accordance with section 515 of Public Law 106-554, the Northeast Fisheries Science Center completed both technical and policy reviews for this report. -

Profile of Nutrition and Hazards of Om-Elkholool (Donax Trunculus) and Gandofly (Ruditapes Decussatus) Clams from Alexandria, Egypt
International Journal For Research In Agricultural And Food Science ISSN: 2208-2719 Profile of Nutrition and Hazards of Om-Elkholool (Donax Trunculus) and Gandofly (Ruditapes Decussatus) Clams From Alexandria, Egypt Sherief Mohammed Sayed Abd-Allah Assistant Professor, Department of Food Hygiene "Meat Hygiene", Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt Email:[email protected] ABSTRACT Clams are delicate nutritious food; however they can harbor potential health hazards. The current work aimed to investigate and compare some of the nutritive criteria and hazards of Om-Elkholool (Donax trunculus) and Gandofly (Ruditapes decussatus) clams sold at Alexandria, Egypt. A total of 46 samples (22 of Om-Elkholool and 24 of Gandofly) were randomly collected from fish retailers during summer of 2017. Samples were analyzed for proximate composition (dry matter, moisture, protein, fat, and ash %). The carbohydrates and energy content was calculated. The count of coliforms, fecal coliforms, E. coli and Cl. perfringenes (MPN/g) was determined. Concentration (mg/kg) of lead and cadmium in 10 randomly selected samples of each type were estimated. The dry matter, moisture, protein, fat, ash and carbohydrates percentages mean values for Om-Elkholool “Om” samples were 30.37±0.22, 69.60±0.21, 8.49±0.14, 1.29±0.03, 18.63±0.09, and 1.99±11, respectively, while for Gandofly “Gd” samples were 16.81±0.21, 83.28±0.2, 8.69±0.13, 1.22±0.03, 3.43±0.09, and 3.37±10, respectively. The gross energy content (Kcal/100g) mean value was 53.55±0.88 for Om and 59.24±0.85 for Gd. -

Impact of Windfarm OWEZ on the Local Macrobenthos Communiy
Impact of windfarm OWEZ on the local macrobenthos community report OWEZ_R_261_T1_20090305 R. Daan, M. Mulder, M.J.N. Bergman Koninklijk Nederlands Instituut voor Zeeonderzoek (NIOZ) This project is carried out on behalf of NoordzeeWind, through a sub contract with Wageningen-Imares Contents Summary and conclusions 3 Introduction 5 Methods 6 Results boxcore 11 Results Triple-D dredge 13 Discussion 16 References 19 Tables 21 Figures 33 Appendix 1 44 Appendix 2 69 Appendix 3 72 Photo’s by Hendricus Kooi 2 Summary and conclusions In this report the results are presented of a study on possible short‐term effects of the construction of Offshore Windfarm Egmond aan Zee (OWEZ) on the composition of the local benthic fauna living in or on top of the sediment. The study is based on a benthic survey carried out in spring 2007, a few months after completion of the wind farm. During this survey the benthic fauna was sampled within the wind farm itself and in 6 reference areas lying north and south of it. Sampling took place mainly with a boxcorer, but there was also a limited programme with a Triple‐D dredge. The occurrence of possible effects was analyzed by comparing characteristics of the macrobenthos within the wind farm with those in the reference areas. A quantitative comparison of these characteristics with those observed during a baseline survey carried out 4 years before was hampered by a difference in sampling design and methodological differences. The conclusions of this study can be summarized as follows: 1. Based on the Bray‐Curtis index for percentage similarity there appeared to be great to very great similarity in the fauna composition of OWEZ and the majority of the reference areas. -

Donacidae - Bivalvia)
Bolm. Zool., Univ. S. P aub 3:121-142, 1978 FUNCTIONAL ANATOMY OF DON AX HANLEY ANUS PHILIPPI 1847 (DONACIDAE - BIVALVIA) Walter Narchi Department o f Zoology University o f São Paulo, Brazil ABSTRACT Donax hanleyanus Philippi 1847 occurs throughout the southern half o f the Brazilian littoral. The main organ systems were studied in the living animal, particular attention being paid to the cilia ry feeding and cleasing mechanisms in the mantle cavity. The anatomy, functioning of the stomach and the ciliary sorting mechanisms are described. The stomach unlike that of almost all species of Donax and like the majority of the Tellinacea belongs to type V, as defined by Purchon, and could be regarded as advanced for the Donacidae. A general comparison has been made between the known species of Donax and some features of Iphigenia brasiliensis Lamarck 1818, also a donacid. INTRODUCTION Very little is known of donacid bivalves from the Brazilian littoral. Except for the publications of Narchi (1972; 1974) on Iphigenia brasiliensis and some ecological and adaptative features on Donax hanleyanus, all references to them are brief descrip tions of the shell and cheklists drawn up from systematic surveys. Beach clams of the genus Donax inhabit intertidal sandy shores in most parts of the world. Donax hanleyanus Philippi 1847 is one of four species occuring through out the Brazilian littoral. Its known range includes Espirito Santo State and the sou thern Atlantic shoreline down to Uruguay (Rios, 1975). According to Penchaszadeh & Olivier (1975) the species occur in the littoral of Argentina. 122 Walter Narchi The species is fairly common in São Paulo, Parana and Santa Catarina States whe re it is used as food by the coastal population (Goffeijé, 1950), and is known as “na- nini” It is known by the name “beguara” (Ihering, 1897) in the Iguape region, but not in S. -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

Estructura Poblacional De Donax Striatus (Bivalvia, Donacidae) En Playa Las Balsas, Gibara, Cuba
ESTRUCTURA POBLACIONAL DE DONAX STRIATUS (BIVALVIA, DONACIDAE) EN PLAYA LAS BALSAS, GIBARA, CUBA Frank A. Ocaña Borrego1*, Alejandro Fernández1, Alexis Silva2, Pedro A. González1 y Yamilet García2 RESUMEN Donax striatus Linné 1767 es una de las dos especies de almejas de playa que se encuentra en el Caribe. Pocas investigaciones se han centrado en describir aspectos sobre la estructura poblacional de esta especie. Una po- blación de D. striatus fue muestreada mensualmente desde febrero del 2008 hasta enero del 2009 en playa Las Balsas, Gibara, Cuba. La población mostró fluctuaciones estacionales de la densidad, presentando los mayores valores en los meses de mayo y octubre del 2008 y enero del 2009. Se encontraron tres picos de reclutamiento: abril-mayo, agosto del 2008 y enero del 2009. Se observó una distribución estratificada por grupos de tallas: los reclutas fueron registrados en los estratos superiores de la playa y los adultos, fundamentalmente, en los es- tratos bajos. La mayor abundancia se localizó en el estrato intermedio. No hubo correlación entre los cambios mensuales de densidad con las temperaturas, ni con las precipitaciones, sin embargo, parece que los cambios en el régimen habitual del oleaje y la elevación del nivel medio del mar tienen influencia sobre el comporta- miento de la abundancia de esta especie. Se propone una hipótesis que combina parámetros morfodinámicos para explicar la distribución vertical de los diferentes componentes de la población. Palabras claves: Bivalvia, Donax, playas arenosas, estructura poblacional, Cuba. ABSTRACT Donax striatus Linné 1767 is one of the two beach clam species that inhabits in the Caribbean. Few in- vestigations have focused on describing aspects related with the population structure of this species. -
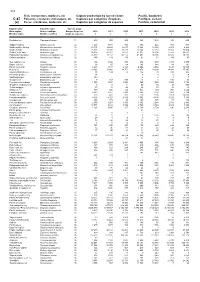
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Bankia Setacea Class: Bivalvia, Heterodonta, Euheterodonta
Phylum: Mollusca Bankia setacea Class: Bivalvia, Heterodonta, Euheterodonta Order: Imparidentia, Myida The northwest or feathery shipworm Family: Pholadoidea, Teredinidae, Bankiinae Taxonomy: The original binomen for Bankia the presence of long siphons. Members of setacea was Xylotrya setacea, described by the family Teredinidae are modified for and Tryon in 1863 (Turner 1966). William Leach distiguished by a wood-boring mode of life described several molluscan genera, includ- (Sipe et al. 2000), pallets at the siphon tips ing Xylotrya, but how his descriptions were (see Plate 394C, Coan and Valentich-Scott interpreted varied. Although Menke be- 2007) and distinct anterior shell indentation. lieved Xylotrya to be a member of the Phola- They are commonly called shipworms (though didae, Gray understood it as a member of they are not worms at all!) and bore into many the Terdinidae and synonyimized it with the wooden structures. The common name ship- genus Bankia, a genus designated by the worm is based on their vermiform morphology latter author in 1842. Most authors refer to and a shell that only covers the anterior body Bankia setacea (e.g. Kozloff 1993; Sipe et (Ricketts and Calvin 1952; see images in al. 2000; Coan and Valentich-Scott 2007; Turner 1966). Betcher et al. 2012; Borges et al. 2012; Da- Body: Bizarrely modified bivalve with re- vidson and de Rivera 2012), although one duced, sub-globular body. For internal anato- recent paper sites Xylotrya setacea (Siddall my, see Fig. 1, Canadian…; Fig. 1 Betcher et et al. 2009). Two additional known syno- al. 2012. nyms exist currently, including Bankia Color: osumiensis, B. -

MAIÊUTICA Ciências Biológicas
MAIÊUTICAMAIÊUTICA CIÊNCIASCIÊNCIAS BIOLÓGICASBIOLOGICAS CENTRO UNIVERSITÁRIO LEONARDO DA VINCI Rodovia BR 470, Km 71, nº 1.040, Bairro Benedito 89130-000 - INDAIAL/SC www.uniasselvi.com.br REVISTA MAIÊUTICA Ciências Biológicas UNIASSELVI 2016 Presidente do Grupo UNIASSELVI Prof. Pedro Jorge Guterres Quintans Graça Reitor da UNIASSELVI Prof. Hermínio Kloch Pró-Reitora de Ensino de Graduação Presencial Profa. Marilda Regiani Olbrzymek Pró-Reitora de Ensino de Graduação a Distância Prof.ª Francieli Stano Torres Pró-Reitor Operacional de Graduação a Distância Prof. Hermínio Kloch Diretor Executivo Unidades Presenciais Prof. Ivan Carlos Hort Diretor de Educação Continuada Prof. Carlos Fabiano Fistarol Editor da Revista Maiêutica Prof. Luis Augusto Ebert Comissão Científica Prof. Alex Giordano Bergmann Prof.ª Claudete Gosczevsk Ciorchetta Prof.ª Claudia Sabrine Brandt Prof.ª Erika Alessandra Rodrigues Prof.ª Joseane Gabrieli Kryzozun Rubin Prof.ª Katia Girardi Dallabona Prof.ª Louise Cristine Franzoi Prof.ª Maquiel Duarte Vidal Prof.ª Renata Joaquim Ferraz Bianco Editoração e Diagramação Jéssica Nauana dos Santos Capa Cleo Schirmann Revisão Final Joice Carneiro Werlang Marcio Kisner Publicação On-line Propriedade do Centro Universitário Leonardo da Vinci Apresentação Apresentamos a você mais uma edição da Revista Maiêutica do Curso de Licenciatura em Ciências Biológicas do Centro universitário Leonardo Da Vinci (UNIASSELVI). A missão da revista é intensificar e divulgar a produção didático-científica de professores e acadêmicos dos cursos que apresentam interesse em publicar artigos na área, cumprindo também o impor- tante papel de tornar acessível à comunidade o que se produz de conhecimento em nosso Centro Universitário. Produzir conhecimento e torná-lo acessível é tarefa que envolve diferentes pessoas, com diferentes formações acadêmicas e de diferentes matrizes teóricas.