An End-To-End Workflow for Quantitative Screening of Multiclass, Multiresidue Veterinary Drugs in Meat Using the Agilent 6470 Triple Quadrupole LC/MS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multi-Residue Automated Turbulent Flow Online LC-MS/MS Method for the Determination of Antibiotics in Milk
Multi-residue Automated Turbulent Flow 63551 Method: Online LC-MS/MS Method for the Determination of Antibiotics in Milk Katerina Bousova, Klaus Mittendorf, Thermo Fisher Scientific Food Safety Response Center, Dreieich, Germany Key Words Transcend TLX, TSQ Quantum Access MAX, Antibiotics, Food Safety, Milk, TurboFlow Technology 1. Schematic of Method Sample Shaking 1. Weigh 500 mg of shaken milk into 2 mL centrifuge tube Sample 500 mg + IS 2. Add 450 µL acetonitrile and 50 µL working IS solution 3. Vortex sample for 5 minutes Extraction 4. Centrifuge sample with 12,000 rpm for 5 minutes For fast screening of antibiotics, microbiological or bioassay techniques are widely used. These techniques 5. Filter sample through 0.45 µm nylon microfilter are not able to distinguish between the different types of antibiotics and provide only a semi-quantitative result Centrifugation and Filtration for the total amount of drug residues. The big drawback is the incidence of false-negative or false-positive results 6. Inject into TLX-LC-MS/MS because of low sensitivity and specificity. However, these screening assays are still very popular and widely used Turbulent Flow LC-MS/MS because of their cost-effectiveness and speed of analysis. For quantitative analysis it is necessary to use instrumental techniques such as LC-MS/MS. This 2. Introduction technique can also be used for screening, and provides Antibiotics are a group of chemicals that are widely used much higher sensitivity and greater specificity. The in animal husbandry primarily for protection of animals use of LC-MS/MS for screening was described in a from disease but also as growth promoters. -

Albany-Molecular-Research-Regulatory
PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF A Abiraterone Malta • Benztropine Mesylate Cedarburg • Adenosine Rozzano - Quinto de' Stampi • • * Betaine Citrate Anhydrous Bon Encontre • Betametasone-17,21- Alcaftadine Spain Spain • • Dipropionate Sterile • Alclometasone-17, 21- Spain Betamethasone Acetate Spain Dipropionate • • Altrenogest Spain • • Betamethasone Base Spain Amphetamine Aspartate Rensselaer Betamethasone Benzoate Spain * Monohydrate Milled • Betamethasone Valerate Amphetamine Sulfate Rensselaer Spain * • Acetate Betamethasone-17,21- Argatroban Rozzano - Quinto de' Stampi Spain • • Dipropionate • • • Atenolol India • • Betamethasone-17-Valerate Spain • • Betamethasone-21- Atracurium Besylate Rozzano - Quinto de' Stampi Spain • Phosphate Disodium Salt • • Bromfenac Monosodium Atropine Sulfate Cedarburg Lodi * • Salt Sesquihydrate • • Azanidazole Lodi Bromocriptine Mesylate Rozzano - Quinto de' Stampi • • • • • Azelastine HCl Rozzano - Quinto de' Stampi • • Budesonide Spain • • Aztreonam Rozzano - Valle Ambrosia • • Budesonide Sterile Spain • • B Bamifylline HCl Bon Encontre • Butorphanol Tartrate Cedarburg • Beclomethasone-17, 21- Spain Capecitabine Lodi Dipropionate • C • 2 *Please contact our Accounts Managers in case you are interested in this API. 3 PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF Dexamethasone-17,21- Carbimazole Bon Encontre Spain • Dipropionate -

(12) Patent Application Publication (10) Pub. No.: US 2010/0221245 A1 Kunin (43) Pub
US 2010O221245A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0221245 A1 Kunin (43) Pub. Date: Sep. 2, 2010 (54) TOPICAL SKIN CARE COMPOSITION Publication Classification (51) Int. Cl. (76) Inventor: Audrey Kunin, Mission Hills, KS A 6LX 39/395 (2006.01) (US) A6II 3L/235 (2006.01) A638/16 (2006.01) Correspondence Address: (52) U.S. Cl. ......................... 424/133.1: 514/533: 514/12 HUSCH BLACKWELL SANDERS LLP (57) ABSTRACT 4801 Main Street, Suite 1000 - KANSAS CITY, MO 64112 (US) The present invention is directed to a topical skin care com position. The composition has the unique ability to treat acne without drying out the user's skin. In particular, the compo (21) Appl. No.: 12/395,251 sition includes a base, an antibacterial agent, at least one anti-inflammatory agent, and at least one antioxidant. The (22) Filed: Feb. 27, 2009 antibacterial agent may be benzoyl peroxide. US 2010/0221 245 A1 Sep. 2, 2010 TOPCAL SKIN CARE COMPOSITION stay of acne treatment since the 1950s. Skin irritation is the most common side effect of benzoyl peroxide and other anti BACKGROUND OF THE INVENTION biotic usage. Some treatments can be severe and can leave the 0001. The present invention generally relates to composi user's skin excessively dry. Excessive use of some acne prod tions and methods for producing topical skin care. Acne Vul ucts may cause redness, dryness of the face, and can actually garis, or acne, is a common skin disease that is prevalent in lead to more acne. Therefore, it would be beneficial to provide teenagers and young adults. -

Horsemen's Information 2016和文TGP 1 薬物修正後 E
[Conditions] 1 Date December 29 (Tue), 2020 2020 Oi Racetrack, Race 10 2 Location TCK, Oi Racetrack 3 Race The 66th Running of Tokyo Daishoten (GI) 4 Eligibility Thoroughbreds, 3 years old & up 5 Full Gate 16 horses 6 Foreign Runners Selected by the selection committee from among the pre-entered horses. 7 Distance 2,000m, 1 1/4 mile (Right-handed, dirt course) 8 Weight 3 years old: 121.5 lbs,4 years old & up: 125.5 lbs, Female: 4.4 lbs less For 3 year-old-horses from the southern hemisphere, reduce 4.4 lbs from the above weight. 9 Purse Unit: 1,000 JPY Prize Purse & Bonus money Running Record 1st place 6th place allowances prize *1 prize *2 1st place 2nd place 3rd place 4th place 5th place or lower Owner 80,000 28,000 16,000 8,000 4,000 300 300 50 1,600 Trainer 880 70 60 50 40 30 30 80 Jockey 120 110 100 80 70 60 30 80 Groom 80 70 60 50 40 30 30 80 Rider 30 30 80 *1 Paid for the runner who broke the previous record and also set the best record during the race. *2 Prize equivalent to the amount listed in the table above is presented. *3 1 USD= 105.88 JPY (As of August,2020) 10 Handling of Late Scratch No allowance is paid in the case of a late scratch (including cancelation of race due to standstill in a starting gate) approved by TCK, Stewards, and Starter. However, if the chairman of the race meeting operation committee deems that the horse is involved in an accident not caused by the horse, the owner is given an running allowance. -

Efficiency of Different Methods of Estrus Synchronization Followed by Fixed Time Artificial Insemination in Persian Downy Does
DOI: 10.21451/1984-3143-AR825 Anim. Reprod., v.14, n.2, p.413-417, Apr./Jun. 2017 Efficiency of different methods of estrus synchronization followed by fixed time artificial insemination in Persian downy does Majid Hashemi1, 2, 3, Mazaher Safdarian2 1Razi Vaccine and Serum Research Institute, Shiraz Branch, Agricultural Research, Education and Extension Organization (AREEO), Shiraz, Iran. 2Animal Science Research Department, Fars Agricultural and Natural Resource Research and Education Center, Agricultural Research, Education and Extension Organization (AREEO), Shiraz, Iran. Abstract estrus synchronization can play an important role for managing production system, allowing the density of For evaluating different methods of long term estrous mating and kidding and production of meat and milk synchronization followed by fixed time artificial during specific times of the year for strategic marketing insemination and to select the most efficient method, and other purposes (Baldassarre and Karatzas, 2004, during the breeding season 160 Persian downy does Zhao et al., 2010). In small ruminants, hormonal estrus were equally allocated to groups (n = 20/group). Estrus synchronization is achieved either by reducing the was synchronized using controlled internal drug release length of the luteal phase of the estrous cycle with devices alone (CIDR) or with equine chorionic prostaglandin F2α or by extending the cycle artificially gonadotropin (CIDR-eCG), intravaginal sponge with exogenous progesterone or more potent impregnated with 45 mg fluorgestone acetate alone progestagens (Hashemi et al., 2006, Abecia et al., (Sponge) or with eCG (Sponge-eCG), subcutaneous 2012). Progestogen administration is common and has auricular implant of 2 mg norgestomet alone (Implant) been used with or without accompanying treatments or with eCG (Implant-eCG) or two intramuscular such as gonadatropins or prostaglandin analogs. -

Reproductive Physiology of the Beef Heifer
Reproductive Physiology of the Beef Heifer Bob L. Larson, DVM, PhD, ACT Hormones Controlling the Estrous Cycle Brain Anterior Hypothalamus Pituitary GnRH n ne e o g FSH r o e rr t s st LH E ge o r P Corpus Uterus Luteum Gn RH Preovulatory Follicle Hypothalamus - Releases small peptides of which GnRH is of direct importance Brain GnRH causes Anterior Hypothalamus pituitary release Pituitary GnRH of FSH and LH ® ® Cystorelin , Factrel , and Fertagyl® are commercially available Ant. Pituitary - FSH stimulates the maturation of 2o follicles LH stimulates maturation of 3o follicles and stimulates estrogen production LH stimulates CL Anterior Pituitary GnRH production of P4 FSH PMSG (eCG) gives LH primarily FSH activity Corpus Luteum hCG gives primarily LH Preovulatory Follicle activity ActionAction ofof GnRHGnRH • Causes release of Luteinizing Hormone (LH) • Ovulation or luteinization • Initiates new follicular wave • CL formation Corpus Luteum - Progesterone prepares the uterus for the egg (d5) P4 acts on the brain to override estrogen to prevent estrus behavior Brain Melengestrol acetate (MGA) Anterior Hypothalamus is synthetic progestogen and Pituitary GnRH natural progesterone is found ne ® o in CIDR insert r e t LH s ge o r P Higher levels of P4 are Corpus Uterus Luteum needed to prevent ovulation than estrus ActionAction ofof ProgesteroneProgesterone • Secreted by CL • Suppress estrus and ovulation • Pregnancy maintenance • “Jump starts” anestrus cows Corpus Luteum Brain Anterior Hypothalamus We control the Pituitary GnRH estrous cycle -

Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians
veterinary sciences Article Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians Carlos Morales-Vallecilla 1, Nicolas Ramírez 1, David Villar 1,*, Maria Camila Díaz 1 , Sandra Bustamante 1 and Duncan Ferguson 2 1 Facultad de Ciencias Agrarias Universidad de Antioquia, Medellín 050010, Colombia; [email protected] (C.M.-V.); [email protected] (N.R.); [email protected] (M.C.D.); [email protected] (S.B.) 2 Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802, USA; [email protected] * Correspondence: [email protected]; Tel.: +57-3178047381 Received: 6 December 2018; Accepted: 5 January 2019; Published: 10 January 2019 Abstract: A questionnaire study was conducted among 131 veterinarians practicing in the city of Medellin, Colombia, to assess views on pain evaluation and management in dogs and cats. When pain recognition and quantification abilities were used as a perceived competence of proper pain assessment, only 83/131 (63.4%, confidence interval (CI) 0.55–0.72) were deemed to have satisfactory skills, with the rest considered to be deficient. There were 49/131 (37.4) veterinarians who had participated in continuing education programs and were more confident assessing pain, with an odds ratio ( standard error) of 2.84 1.15 (p = 0.01; CI 1.27–6.32). In addition, the odds of using ± ± pain scales was 4.28 2.17 (p < 0.01, CI 1.58–11.55) greater if they had also participated in continuing ± education programs. The term multimodal analgesia was familiar to 77 (58.7%) veterinarians who also claimed to use more than one approach to pain control. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Summary of Product Characteristics 1. Name Of
Revised: July 2020 AN: 00391/2020 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Chronogest CR, 20 mg controlled release vaginal sponge for sheep. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each polyester polyurethane sponge contains Active substance(s) Flugestone acetate, 20 mg. List of excipients Excipients qsp 1 sponge. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Vaginal sponge. White cylindrical polyester polyurethane foam equipped with string. 4. CLINICAL PARTICULARS 4.1 Target species Sheep (ewes and ewe-lambs). 4.2 Indications for use In ewes and ewe lambs, in combination with PMSG (Pregnant Mare Serum Gonadotrophin) - Induction and synchronization of oestrus and ovulation (non cycling ewes during seasonal anoestrus and ewe lambs). - Synchronization of oestrus and ovulation (cycling ewes and ewe-lambs). 4.3 Contraindications Please refer to section 4.7 and section 4.8. 4.4 Special warnings None. Page 1 of 5 Revised: July 2020 AN: 00391/2020 4.5 Special precautions for use (i) Special precautions for use in animals - The repeated treatment with the product combined with PMSG may trigger the appearance of PMSG antibodies in some ewes. This in turn may affect the time of ovulation and result in reduced fertility when combined with fixed time artificial insemination at 55h following sponge removal. - The repeated use of sponges within one year has not been studied. - The use of a vaginal applicator designed for ewes or ewe lambs is recommended to correctly insert sponges and to avoid vaginal injuries. (ii) Special precautions to be taken by the person administering the medicinal product to animals - Direct contact with the skin should be avoided. -

Transdermal Drug Delivery Device Including An
(19) TZZ_ZZ¥¥_T (11) EP 1 807 033 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61F 13/02 (2006.01) A61L 15/16 (2006.01) 20.07.2016 Bulletin 2016/29 (86) International application number: (21) Application number: 05815555.7 PCT/US2005/035806 (22) Date of filing: 07.10.2005 (87) International publication number: WO 2006/044206 (27.04.2006 Gazette 2006/17) (54) TRANSDERMAL DRUG DELIVERY DEVICE INCLUDING AN OCCLUSIVE BACKING VORRICHTUNG ZUR TRANSDERMALEN VERABREICHUNG VON ARZNEIMITTELN EINSCHLIESSLICH EINER VERSTOPFUNGSSICHERUNG DISPOSITIF D’ADMINISTRATION TRANSDERMIQUE DE MEDICAMENTS AVEC COUCHE SUPPORT OCCLUSIVE (84) Designated Contracting States: • MANTELLE, Juan AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Miami, FL 33186 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • NGUYEN, Viet SK TR Miami, FL 33176 (US) (30) Priority: 08.10.2004 US 616861 P (74) Representative: Awapatent AB P.O. Box 5117 (43) Date of publication of application: 200 71 Malmö (SE) 18.07.2007 Bulletin 2007/29 (56) References cited: (73) Proprietor: NOVEN PHARMACEUTICALS, INC. WO-A-02/36103 WO-A-97/23205 Miami, FL 33186 (US) WO-A-2005/046600 WO-A-2006/028863 US-A- 4 994 278 US-A- 4 994 278 (72) Inventors: US-A- 5 246 705 US-A- 5 474 783 • KANIOS, David US-A- 5 474 783 US-A1- 2001 051 180 Miami, FL 33196 (US) US-A1- 2002 128 345 US-A1- 2006 034 905 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -
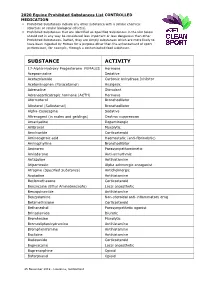
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

PT Animal Feed Drugs
Animal Feed Drugs • 26 labs/facilities reported at least one drug between 2016 to April 2018 • 2017 Lab Method’s Need Survey (based on the 2016 Compendium) – 41 drugs were checked by respondents. Up to 23 labs indicated that they run or were interested in adding certain drugs. • 44 Drugs or Drug Combination are listed in the 2018 Compendium • 66 AAFCO PT Method Codes – Mix of some current drugs and drugs no longer listed in the compendium. • Only 19 drugs reported from the current method code list between 2016 to April 2018 © 2018 Association of American Feed Control Officials (AAFCO) 1800 S. Oak Street, Suite 100, Champaign, IL 61820-6974 Methods Needs Survey 2017 vs 30 25 20 15 Chlortet 10 Decoquinate 5 0 Lasalocid Amprolium Lasalocid Arsanilic Acid Bacitracin Sodium Bacitracin Zinc Carbadox Chlortetracycline/Penicillin/Sulfamethazine Clopidol Cyromazine Reported Diclazuril Diflubenzuron Ethopabate Fenbendazole Furazolidone Hygromycin B Ivermecti n Oxytet Lasalocid (Max per Round) LeVamisole Maduramicin Menadione (form) Monensin Narasin © 2018 MethodsAssociation Needs ofSurVey American - 2017 Feed Control OfficialsNeomycin (AAFCO) Nicarbazin 1800 S. Oak Street, Suite 100, Champaign, IL 61820 Nitrofurazone NoVobiocin Ormetoprim Penicillin Piperazine Pyrantel Tartrate Tylosin Max Reported from 2016 to 2018 (April) Ractopamine Hydrochloride Robenidine Hyrochloride (S)-Methoprene Semduramicin Spectinomycin Sulfadimethoxine Sulfadimethoxine and Ormetoprim 5:3 Sulfanitran -6974 Sulfathiazole Thiabendazole Til micosin Tylosin/Sulfamethazine Tyl Valosin Tartarate Zilpaterol Zoalene • VFD drugs and/or Feeds • Residues – We’ve added residue levels of drugs. We have between 1 to 16 labs report in a round. • Why are drugs not analyzed? • Not required by program? • Regional? • Economic? • Lack of equipment? • Specific drug not used internationally? • Melegestrol acetate –Are labs planning on analyzing steroids? © 2018 Association of American Feed Control Officials (AAFCO) 1800 S.