Horsemen's Information 2016和文TGP 1 薬物修正後 E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solid Solution Compositions and Use in Chronic Inflammation
(19) *EP003578172A1* (11) EP 3 578 172 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 11.12.2019 Bulletin 2019/50 A61K 9/14 (2006.01) A61K 47/14 (2017.01) A61K 47/44 (2017.01) A61K 31/12 (2006.01) (2006.01) (2006.01) (21) Application number: 19188174.7 A61K 31/137 A61K 31/167 A61K 31/192 (2006.01) A61K 31/216 (2006.01) (2006.01) (2006.01) (22) Date of filing: 14.01.2014 A61K 31/357 A61K 31/366 A61K 31/4178 (2006.01) A61K 31/4184 (2006.01) A61K 31/522 (2006.01) A61K 31/616 (2006.01) A61K 31/196 (2006.01) (84) Designated Contracting States: • BREW, John AL AT BE BG CH CY CZ DE DK EE ES FI FR GB London, Greater London EC2Y 8AD (GB) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • REILEY, Richard Robert PL PT RO RS SE SI SK SM TR London, Greater London EC2Y 8AD (GB) • CAPARRÓS-WANDERLEY, Wilson (30) Priority: 14.01.2013 US 201361752356 P London, Greater London EC2Y 8AD (GB) 04.02.2013 US 201361752309 P (74) Representative: Clements, Andrew Russell Niel et (62) Document number(s) of the earlier application(s) in al accordance with Art. 76 EPC: Schlich 14702460.8 / 2 925 367 9 St Catherine’s Road Littlehampton, West Sussex BN17 5HS (GB) (71) Applicant: InFirst Healthcare Limited London EC2Y 8AD (GB) Remarks: This application was filed on 24-07-2019 as a (72) Inventors: divisional application to the application mentioned • BANNISTER, Robin Mark under INID code 62. -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

Use of Aspirin and Nsaids to Prevent Colorectal Cancer
Evidence Synthesis Number 45 Use of Aspirin and NSAIDs to Prevent Colorectal Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-02-0021 Prepared by: University of Ottawa Evidence-based Practice Center at The University of Ottawa, Ottawa Canada David Moher, PhD Director Investigators Alaa Rostom, MD, MSc, FRCPC Catherine Dube, MD, MSc, FRCPC Gabriela Lewin, MD Alexander Tsertsvadze, MD Msc Nicholas Barrowman, PhD Catherine Code, MD, FRCPC Margaret Sampson, MILS David Moher, PhD AHRQ Publication No. 07-0596-EF-1 March 2007 This report is based on research conducted by the University of Ottawa Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0021). Funding was provided by the Centers for Disease Control and Prevention. The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help clinicians, employers, policymakers, and others make informed decisions about the provision of health care services. This report is intended as a reference and not as a substitute for clinical judgment. This document is in the public domain and may be used and reprinted without permission except those copyrighted materials noted for which further reproduction is prohibited without the specific permission of copyright holders. -

The 2006 Prohibited List International Standard
The World Anti-Doping Code THE 2006 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2006. THE 2006 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2006 The use of any drug should be limited to medically justified indications SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β-dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone (17β-hydroxy-17α- methylandrosta-1,4-dien-3-one); methandriol; methasterone (2α, 17α- dimethyl-5α-androstane-3-one-17β-ol); methyldienolone -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

The Involvement of Endoplasmic Reticulum Stress in the Suppression of Colorectal Tumorigenesis by Tolfenamic Acid
Published OnlineFirst October 8, 2013; DOI: 10.1158/1940-6207.CAPR-13-0220 Cancer Prevention Research Article Research The Involvement of Endoplasmic Reticulum Stress in the Suppression of Colorectal Tumorigenesis by Tolfenamic Acid Xiaobo Zhang1,2, Seong-Ho Lee3, Kyung-Won Min2, Michael F. McEntee2, Jin Boo Jeong3, Qingwang Li1, and Seung Joon Baek2 Abstract The nonsteroidal anti-inflammatory drug tolfenamic acid has been shown to suppress cancer cell growth and tumorigenesis in different cancer models. However, the underlying mechanism by which tolfenamic acid exerts its antitumorigenic effect remains unclear. Previous data from our group and others indicate that tolfenamic acid alters expression of apoptosis- and cell-cycle arrest–related genes in colorectal cancer cells. þ Here, we show that tolfenamic acid markedly reduced the number of polyps and tumor load in APCmin/ mice, accompanied with cyclin D1 downregulation in vitro and in vivo. Mechanistically, tolfenamic acid promotes endoplasmic reticulum (ER) stress, resulting in activation of the unfolded protein response (UPR) signaling pathway, of which PERK-mediated phosphorylation of eukaryotic translation initiation factor 2a (eIF2a) induces the repression of cyclin D1 translation. Moreover, the PERK-eIF2a-ATF4 branch of the UPR pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells, as silencing ATF4 attenuates tolfenamic acid-induced apoptosis. Taken together, these results suggest ER stress is involved in tolfenamic acid-induced inhibition of colorectal cancer cell growth, which could contribute to antitumor- igenesis in a mouse model. Cancer Prev Res; 6(12); 1–11. Ó2013 AACR. Introduction and has received much attention since the inhibition of Colorectal cancer is the third-leading cause of cancer- COX-2 activity produces adverse effects (5). -

4 Supplementary File
Supplemental Material for High-throughput screening discovers anti-fibrotic properties of Haloperidol by hindering myofibroblast activation Michael Rehman1, Simone Vodret1, Luca Braga2, Corrado Guarnaccia3, Fulvio Celsi4, Giulia Rossetti5, Valentina Martinelli2, Tiziana Battini1, Carlin Long2, Kristina Vukusic1, Tea Kocijan1, Chiara Collesi2,6, Nadja Ring1, Natasa Skoko3, Mauro Giacca2,6, Giannino Del Sal7,8, Marco Confalonieri6, Marcello Raspa9, Alessandro Marcello10, Michael P. Myers11, Sergio Crovella3, Paolo Carloni5, Serena Zacchigna1,6 1Cardiovascular Biology, 2Molecular Medicine, 3Biotechnology Development, 10Molecular Virology, and 11Protein Networks Laboratories, International Centre for Genetic Engineering and Biotechnology (ICGEB), Padriciano, 34149, Trieste, Italy 4Institute for Maternal and Child Health, IRCCS "Burlo Garofolo", Trieste, Italy 5Computational Biomedicine Section, Institute of Advanced Simulation IAS-5 and Institute of Neuroscience and Medicine INM-9, Forschungszentrum Jülich GmbH, 52425, Jülich, Germany 6Department of Medical, Surgical and Health Sciences, University of Trieste, 34149 Trieste, Italy 7National Laboratory CIB, Area Science Park Padriciano, Trieste, 34149, Italy 8Department of Life Sciences, University of Trieste, Trieste, 34127, Italy 9Consiglio Nazionale delle Ricerche (IBCN), CNR-Campus International Development (EMMA- INFRAFRONTIER-IMPC), Rome, Italy This PDF file includes: Supplementary Methods Supplementary References Supplementary Figures with legends 1 – 18 Supplementary Tables with legends 1 – 5 Supplementary Movie legends 1, 2 Supplementary Methods Cell culture Primary murine fibroblasts were isolated from skin, lung, kidney and hearts of adult CD1, C57BL/6 or aSMA-RFP/COLL-EGFP mice (1) by mechanical and enzymatic tissue digestion. Briefly, tissue was chopped in small chunks that were digested using a mixture of enzymes (Miltenyi Biotec, 130- 098-305) for 1 hour at 37°C with mechanical dissociation followed by filtration through a 70 µm cell strainer and centrifugation. -

Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians
veterinary sciences Article Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians Carlos Morales-Vallecilla 1, Nicolas Ramírez 1, David Villar 1,*, Maria Camila Díaz 1 , Sandra Bustamante 1 and Duncan Ferguson 2 1 Facultad de Ciencias Agrarias Universidad de Antioquia, Medellín 050010, Colombia; [email protected] (C.M.-V.); [email protected] (N.R.); [email protected] (M.C.D.); [email protected] (S.B.) 2 Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802, USA; [email protected] * Correspondence: [email protected]; Tel.: +57-3178047381 Received: 6 December 2018; Accepted: 5 January 2019; Published: 10 January 2019 Abstract: A questionnaire study was conducted among 131 veterinarians practicing in the city of Medellin, Colombia, to assess views on pain evaluation and management in dogs and cats. When pain recognition and quantification abilities were used as a perceived competence of proper pain assessment, only 83/131 (63.4%, confidence interval (CI) 0.55–0.72) were deemed to have satisfactory skills, with the rest considered to be deficient. There were 49/131 (37.4) veterinarians who had participated in continuing education programs and were more confident assessing pain, with an odds ratio ( standard error) of 2.84 1.15 (p = 0.01; CI 1.27–6.32). In addition, the odds of using ± ± pain scales was 4.28 2.17 (p < 0.01, CI 1.58–11.55) greater if they had also participated in continuing ± education programs. The term multimodal analgesia was familiar to 77 (58.7%) veterinarians who also claimed to use more than one approach to pain control. -
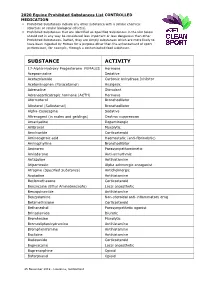
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

Antioxidant and Anti-Inflammatory Agents Mitigate Pathology in A
Human Molecular Genetics, 2015, Vol. 24, No. 14 3918–3928 doi: 10.1093/hmg/ddv122 Advance Access Publication Date: 9 April 2015 Original Article ORIGINAL ARTICLE Antioxidant and anti-inflammatory agents mitigate pathology in a mouse model of pseudoachondroplasia Karen L. Posey1,*, Francoise Coustry1, Alka C. Veerisetty1, Downloaded from Mohammad Hossain1, Joseph L. Alcorn1 and Jacqueline T. Hecht1,2 1Department of Pediatrics, University of Texas Medical School at Houston, Houston, TX, USA and 2Shriners Hospital for Children, Houston, TX, USA http://hmg.oxfordjournals.org/ *To whom correspondence should be addressed. Tel: +1 7135005786; Fax: +1 7135005689; Email: [email protected] Abstract Pseudoachondroplasia (PSACH), a severe short-limb dwarfing condition, results from mutations that cause misfolding of the cartilage oligomeric matrix protein (COMP). Accumulated COMP in growth plate chondrocytes activates endoplasmic reticulum stress, leading to inflammation and chondrocyte death. Using a MT-COMP mouse model of PSACH that recapitulates the molecular and clinical PSACH phenotype, we previously reported that oxidative stress and inflammation play important and at HAM-TMC Library on October 13, 2015 unappreciated roles in PSACH pathology. In this study, we assessed the ability of antioxidant and anti-inflammatory agents to affect skeletal and cellular pathology in our mouse model of PSACH. Treatment of MT-COMP mice with aspirin or resveratrol from birth to P28 decreased mutant COMP intracellular retention and chondrocyte cell death, and restored chondrocyte proliferation. Inflammatory markers associated with cartilage degradation and eosinophils were present in the joints of untreated juvenile MT-COMP mice, but were undetectable in treated mice. Most importantly, these treatments resulted in significantly increased femur length.