Assessment of the Efficacy of Firocoxib and Robenacoxib
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Horsemen's Information 2016和文TGP 1 薬物修正後 E
[Conditions] 1 Date December 29 (Tue), 2020 2020 Oi Racetrack, Race 10 2 Location TCK, Oi Racetrack 3 Race The 66th Running of Tokyo Daishoten (GI) 4 Eligibility Thoroughbreds, 3 years old & up 5 Full Gate 16 horses 6 Foreign Runners Selected by the selection committee from among the pre-entered horses. 7 Distance 2,000m, 1 1/4 mile (Right-handed, dirt course) 8 Weight 3 years old: 121.5 lbs,4 years old & up: 125.5 lbs, Female: 4.4 lbs less For 3 year-old-horses from the southern hemisphere, reduce 4.4 lbs from the above weight. 9 Purse Unit: 1,000 JPY Prize Purse & Bonus money Running Record 1st place 6th place allowances prize *1 prize *2 1st place 2nd place 3rd place 4th place 5th place or lower Owner 80,000 28,000 16,000 8,000 4,000 300 300 50 1,600 Trainer 880 70 60 50 40 30 30 80 Jockey 120 110 100 80 70 60 30 80 Groom 80 70 60 50 40 30 30 80 Rider 30 30 80 *1 Paid for the runner who broke the previous record and also set the best record during the race. *2 Prize equivalent to the amount listed in the table above is presented. *3 1 USD= 105.88 JPY (As of August,2020) 10 Handling of Late Scratch No allowance is paid in the case of a late scratch (including cancelation of race due to standstill in a starting gate) approved by TCK, Stewards, and Starter. However, if the chairman of the race meeting operation committee deems that the horse is involved in an accident not caused by the horse, the owner is given an running allowance. -

Non-Steroidal Anti-Inflammatory Drugs As Chemopreventive Agents: Evidence from Cancer Treatment in Domestic Animals
Annual Research & Review in Biology 26(1): 1-13, 2018; Article no.ARRB.40829 ISSN: 2347-565X, NLM ID: 101632869 Non-Steroidal Anti-Inflammatory Drugs as Chemopreventive Agents: Evidence from Cancer Treatment in Domestic Animals Bianca F. Bishop1 and Suong N. T. Ngo1* 1School of Animal and Veterinary Sciences, The University of Adelaide, Roseworthy, SA 5371, Australia. Authors’ contributions This work was carried out in collaboration between both authors. Author BFB performed the collection and analysis of the data. Author SNTN designed the study, managed the analyses and interpretation of the data and prepared the manuscript. Both authors read and approved the final manuscript. Article Information DOI: 10.9734/ARRB/2018/40829 Editor(s): (1) David E. Martin, Martin Pharma Consulting, LLC, Shawnee, OK, USA. (2) George Perry, Dean and Professor of Biology, University of Texas at San Antonio, USA. Reviewers: (1) Fulya Ustun Alkan, Istanbul University, Turkey. (2) Thompson Akinbolaji, USA. (3) Ramesh Gurunathan, Sunway Medical Center, Malaysia. (4) Mohamed Ahmed Mohamed Nagy Mohamed, El Minia Hospital, Egypt. Complete Peer review History: http://www.sciencedomain.org/review-history/24385 Received 10th February 2018 Accepted 21st April 2018 Review Article Published 30th April 2018 ABSTRACT Aims: This study aims to systematically review currently available data on the use of non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of cancer in domestic animals to evaluate the efficacy of different treatment protocols and to suggest further recommendations for future study. Methodology: Literature data on the use of NSAIDs in domestic animals as chemo-preventive agents in the last decade were collected and critically reviewed. -

Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians
veterinary sciences Article Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians Carlos Morales-Vallecilla 1, Nicolas Ramírez 1, David Villar 1,*, Maria Camila Díaz 1 , Sandra Bustamante 1 and Duncan Ferguson 2 1 Facultad de Ciencias Agrarias Universidad de Antioquia, Medellín 050010, Colombia; [email protected] (C.M.-V.); [email protected] (N.R.); [email protected] (M.C.D.); [email protected] (S.B.) 2 Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802, USA; [email protected] * Correspondence: [email protected]; Tel.: +57-3178047381 Received: 6 December 2018; Accepted: 5 January 2019; Published: 10 January 2019 Abstract: A questionnaire study was conducted among 131 veterinarians practicing in the city of Medellin, Colombia, to assess views on pain evaluation and management in dogs and cats. When pain recognition and quantification abilities were used as a perceived competence of proper pain assessment, only 83/131 (63.4%, confidence interval (CI) 0.55–0.72) were deemed to have satisfactory skills, with the rest considered to be deficient. There were 49/131 (37.4) veterinarians who had participated in continuing education programs and were more confident assessing pain, with an odds ratio ( standard error) of 2.84 1.15 (p = 0.01; CI 1.27–6.32). In addition, the odds of using ± ± pain scales was 4.28 2.17 (p < 0.01, CI 1.58–11.55) greater if they had also participated in continuing ± education programs. The term multimodal analgesia was familiar to 77 (58.7%) veterinarians who also claimed to use more than one approach to pain control. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -
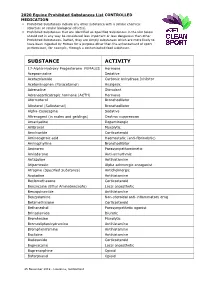
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

An End-To-End Workflow for Quantitative Screening of Multiclass, Multiresidue Veterinary Drugs in Meat Using the Agilent 6470 Triple Quadrupole LC/MS
Application Note Food Testing & Agriculture An End-To-End Workflow for Quantitative Screening of Multiclass, Multiresidue Veterinary Drugs in Meat Using the Agilent 6470 Triple Quadrupole LC/MS Authors Abstract Siji Joseph, Aimei Zou, Chee A comprehensive LC/MS/MS workflow was developed for targeted screening or Sian Gan, Limian Zhao, and quantitation of 210 veterinary drug residues in animal muscle prepared for human Patrick Batoon consumption, with the intention to accelerate and simplify routine laboratory testing. Agilent Technologies, Inc. The workflow ranged from sample preparation through chromatographic separation, MS detection, data processing and analysis, and report generation. The workflow performance was evaluated using three muscle matrices—chicken, pork, and beef— and was assessed on two different Agilent triple quadrupole LC/MS models (an Agilent 6470 and a 6495C triple quadrupole LC/MS). A simple sample preparation protocol using Agilent Captiva EMR—Lipid cartridges provided efficient extraction and matrix cleanup. A single chromatographic method using Agilent InfinityLab Poroshell 120 EC-C18 columns with a 13-minute method delivered acceptable separation and retention time distribution across the elution window for reliable triple quadrupole detection and data analysis. Workflow performance was evaluated based on evaluation of limit of detection (LOD), limit of quantitation (LOQ), calibration curve linearity, accuracy, precision, and recovery, using matrix-matched spike samples for a range from 0.1 to 100 μg/L. Calibration curves were plotted from LOQ to 100 μg/L, where all analytes demonstrated linearity R2 >0.99. Instrument method accuracy values were within 73 to 113%. Target analytes response and retention time %RSD values were ≤19% and ≤0.28% respectively. -

(12) United States Patent (10) Patent No.: US 8,722,636 B2 Rock Et Al
USOO8722636B2 (12) United States Patent (10) Patent No.: US 8,722,636 B2 Rock et al. (45) Date of Patent: May 13, 2014 (54) ANIMAL TREATMENTS 2004/0248942 A1* 12/2004 Hepburn et al. .............. 514,338 2007/0042023 A1 2/2007 Puri et al. 2007. O1841.06 A1 8/2007 Schellenger et al. (75) Inventors: s G "Eby, S.S); 2007/0275058 A1* 11/2007 Tanaka et al. ................. 424/465 ark Ridall, Princeton, NJ (US) 2008, 0096971 A1* 4/2008 Baxter et al. .................. 514,646 (73) Assignee: New Market Pharmaceuticals, LLC, OTHER PUBLICATIONS Princeton, NJ (US) Glossary of medical education terms, Institute of International Medi (*) Notice: Subject to any disclaimer, the term of this cal Education. http://www.iime.org/glossary.htm Accessed on Jan. patent is extended or adjusted under 35 2013.* U.S.C. 154(b) by 0 days. Chubineh et al. Proton pump inhibitors: the good, the bad, and the unwanted. South Med J 105:613-618, Nov. 2012.* (21) Appl. No.: 13/343,692 www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarian Newsletter/ucm100268.htm, Mar/Apr. 2003. (22) Filed: Jan. 4, 2012 Veterinary Compounding Brochure, American Veterinary Medical 9 Association (AVMA) Jun. 2001. O O Nagar, Mona et al., “Formulation, Evaluation and Comparison of (65) Prior Publication Data Fast-Dissolving Tablet of Nimesulide by Using Crospovidone as US 2012/O196819 A1 Aug. 2, 2012 Superdisintegrant.” International Journal of Pharmaceutical Sciences and Drug Research. 2009, 1(3), pp. 172-175. Related U.S. Application Data Panigrahi. R et al., “A Review On Fast Dissolving Tablets.” WebmedCentral Pharmaceutical Sciences. -

Robenacoxib Shows Efficacy for the Treatment of Chronic Degenerative Joint Disease-Associated Pain in Cats
www.nature.com/scientificreports OPEN Robenacoxib shows efcacy for the treatment of chronic degenerative joint disease‑associated pain in cats: a randomized and blinded pilot clinical trial Derek Adrian1,9, Jonathan N. King2, Rudolph S. Parrish3,10, Stephen B. King3, Steven C. Budsberg4, Margaret E. Gruen1,5,6 & B. Duncan X. Lascelles1,6,7,8* The main objective of this pilot clinical trial was to evaluate outcome measures for the assessment of the nonsteroidal anti‑infammatory drug (NSAID) robenacoxib in cats with degenerative joint disease‑ associated pain (DJD‑pain). Otherwise healthy cats (n = 109) with DJD‑pain entered a parallel group, randomized, blinded clinical trial. Cats received placebo (P) or robenacoxib (R) for two consecutive 3‑week periods. Treatment groups were PP, RR, and RP. Actimetry and owner‑assessment data were collected. Data were analyzed using mixed‑efects and generalized mixed‑efects linear models. Activity data showed high within‑cat and between‑cat variability, and 82.4% of the values were zero. Compared to placebo, mean total activity was higher (5.7%) in robenacoxib‑treated cats (p = 0.24); for the 80th percentile of activity, more robenacoxib‑treated cats had a > 10% increase in activity after 3 (p = 0.046) and 6 weeks (p = 0.026). Robenacoxib treatment signifcantly decreased owner‑ assessed disability, (p = 0.01; 49% reduction in disability; efect size ~ 0.3), and improved temperament (p = 0.0039) and happiness (p = 0.021) after 6 weeks. More robenacoxib‑treated cats were successes at 6 weeks (p = 0.018; NNT: 3.8). Adverse efect frequencies were similar across groups. -

HOSPITAL WISH LIST Your Donation Supports the Care and Rehabilitation of Injured, Orphaned, Or Displaced Wildlife
HOSPITAL WISH LIST Your donation supports the care and rehabilitation of injured, orphaned, or displaced wildlife ADMINISTRATION ANIMAL HOUSING & BEDDING Copy paper: All colors and sizes, Letter and Legal Acrylic Critter Keepers/Pet Taxis most needed Aquarium tanks: 10 gallon preferred Masking tape: 1 or 2-inch wide Cages: Large, tall, wire cages for cats, ferrets, parrots Power strips with surge protection Fleece Box fans Gloves: Leather, welding, or light duty Calculators Heating Pads: No auto-shut-off, Sunbeam® Model Clear sheet protectors 756-500 Correction fluid or correction tape Heating Pads: Heavy-duty, outdoor (small animal) Duct tape Aspen shavings Extension cords and plug covers Baby blankets: All sizes New or used Hot laminator sheets Bolt snaps: Heavy-duty Markers: Dry erase, Permanent Bulbs: 75 watt infrared heat Pens Bulbs: ZooMed® 10.0 UVB Scissors Bungee cords with metal hooks Carabiner clips CLEANING Floor mats: Rubber anti-slip, Astroturf® Bleach: Unscented Hand warmers: HotHands Brooms & Dust pans Kiddie/Wading pools: Plastic, hard-walled Dish soap: Unscented, Antibiotic Litter pan covers for enclosed litter boxes Laundry detergent: Unscented, HE Litter pans: All Sizes, clean Paper towels Newspaper: Preferably without ad pages, no staples Tissues Pillowcases: All sizes, new or used Toilet paper Placemats: Canvas (for hammocks) Trash bags: 39 gallon and larger, All sizes, unscented Sheets: All sizes, New or used Floor squeegees Stock Tanks: 50-300 gallon metal or rubbermaid Hand sanitizer: Non-alcohol based preferred -

A. List of Prohibited Substances 1
Reviewed 09.05.17 List over prohibited substances and withdrawal times in Scandinavia, valid from May 20th, 2017 This list has been developed in collaboration with the other Scandinavian countries through NEMAC (Nordic Equine Medication and Anti-doping Committee) The List of prohibited substances and withdrawal times consists of the A-list, listing substances and treatment methods that are absolutely prohibited for horses, and the B-list, listing substances that are prohibited in competition; the withdrawal times for these substances as well as treatment methods with withdrawal times. The list may be reviewed several times per year. This list is valid starting from May 20th, 2017 and is enforced until a new list takes effect. A valid list of withdrawal times can also be found at any time on DNT’s official website, www.travsport.no.ST’s official website, www.travsport.se, and DTC`s official website www.trav.dk Health certificate and keeping of medical records The trainer is responsible for ensuring that any treatment that requires a withdrawal time is listed in the horse’s health certificate. The start and end dates of the treatment, the name of the treatment/medication/active substance, amount given, method of administration, withdrawal time as well as the name of the veterinarian or other person responsible for the treatment must all be listed in the health certificate in accordance with DNT’s Doping Regulations 2017 § 4. Passport and health certificate should be brought with the horse at all times. Omitting, incompletely or improperly listing treatments in the health certificate constitutes a breach of the Doping Regulations. -

Effects of Osteoarthritis and Chronic Pain Management for Companion Animals Rebecca A
Southern Illinois University Carbondale OpenSIUC Research Papers Graduate School 2014 Effects of Osteoarthritis and Chronic Pain Management for Companion Animals Rebecca A. Cason Southern Illinois University Carbondale, [email protected] Follow this and additional works at: http://opensiuc.lib.siu.edu/gs_rp Recommended Citation Cason, Rebecca A., "Effects of Osteoarthritis and Chronic Pain Management for Companion Animals" (2014). Research Papers. Paper 521. http://opensiuc.lib.siu.edu/gs_rp/521 This Article is brought to you for free and open access by the Graduate School at OpenSIUC. It has been accepted for inclusion in Research Papers by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. EFFECTS OF OSTEOARTHRITIS AND CHRONIC PAIN MANAGEMENT FOR COMPANION ANIMALS By Rebecca A. Cason B.S., Southern Illinois University Carbondale, 2012 A Research Paper Submitted in Partial Fulfillment of the Requirements for the Master of Science. Department of Animal Science, Food and Nutrition In the Graduate School Southern Illinois University Carbondale May 2014 RESEARCH PAPER APPROVAL EFFECTS OF OSTEOARTHRITIS AND CHRONIC PAIN MANAGEMENT FOR COMPANION ANIMALS By Rebecca A. Cason A Research Paper Submitted in Partial Fulfillment of the Requirements for the Degree of Masters in Science in the field of Animal Science Approved by: Rebecca Atkinson, Chair Amer AbuGhazaleh Nancy Henry Graduate School Southern Illinois University Carbondale March 18, 2014 TABLE OF CONTENTS Page LIST OF FIGURES.……………………………………………………………………………....ii -

Robenacoxib-Cats-2019 (Onsior)
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided by prescribing veterinarian] Robenacoxib (Cats) (roe-ben-ah-cox-ib) Description: Nonsteroidal Antiinflammatory Drug (NSAID) Other Names for this Medication: Onsior® Common Dosage Forms: Veterinary: 6 mg (yeast flavored) tablets. Human: None. This information sheet does not contain all available information for this medication. It is to help answer commonly asked questions and help you give the medication safely and effectively to your animal. If you have other questions or need more information about this medication, contact your veterinarian or pharmacist. Key Information NSAID approved for cats for short-term treatment (up to 3 days) of pain. Giving this medicine for longer periods of time may increase risks. May be given with or without food, but giving with food may help prevent stomach upset. Do not split tablets. When the drug is used as directed on the label, most cats tolerate the drug well. Side effects are usually mild, but serious side effects can occur. If any of the following are seen, stop the drug and contact your veterinarian immediately: Decrease in appetite, vomiting, changes in bowel movements (eg, diarrhea, constipation, color), changes in drinking or urination habits (eg, frequency, amounts, smell), changes in behavior (eg, depression, restlessness), seizures, or jaundice (yellowing of gums, skin, or whites of the eyes). Pregnant women, especially those close to term, should be very careful when handling this medicine. How is this medication useful? The FDA (U.S.