Evidence for a Southward Autumn Migration of Nocturnal Noctuid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butterflies and Moths of Ada County, Idaho, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

New Moths from Texas (Noctuidae, Tortricidae)
1968 Joltrnal of the Lepidopterists' Societu 133 NEW MOTHS FROM TEXAS (NOCTUIDAE, TORTRICIDAE) ANDRE BLANCHARD 3023 Underwood, Houston, Texas As a retirement hobby, I decided, six years ago, to catalog the moths of Texas. My wife and I have been collecting moths all over Texas for the last four years. As the work progressed, I came to realize that I may have to settle, more modestly, for a "Contributions Toward A Catalog of the Moths of Texas." The number of species in my collection which had apparently never been taken in Texas is quite large, and the number of those which seem to be new to science, particularly from the mountain ranges and desert areas of West Texas, is much larger than I ever expected. I have been fortunate in interesting several specialists in describing some of these new species: Dr. C. L. Hogue (196S), Mr. McElvare ( 1966), Dr. E. L. Todd (1966) have described three. Difficult cases are now and, in the future, will be submitted to experts. I have de scribed the male of a fourth species (1966), the female of which was described by Dr. F. H. Hindge (1966). While getting more material for my intcnded catalog, I shall describe as many of the new species as I can name without becoming guilty of adding to the confusion which already exists in some genera. In the present paper, I describe six new noctuids and one tortricid species. All types were collected by A. &. M. E. Blanchard. Acronicta vallis cola Blanchard, new species (PI. I, fig. J; PI. -

GMS News Autumn 2019 Weeks 28-36
GMS News Autumn 2019 Weeks 28-36 Contents Editorial Norman Lowe 1 Overview GMS 2019 4th Quarter Evan Lynn 2 My move to the “Dark Side” David Baker 12 Another snippet! (VC73) Rhian & Adam Davies 17 Emperors, admirals and chimney sweepers, a book review Peter Major 15 GMS collaboration with Cairngorms Connect Stephen Passey 22 How and when to release trapped moths? Norman Lowe 23 Garden Moth Scheme South Wales Conference Norman Lowe 23 Clifden Nonpareil Michael Sammes 24 Tailpiece Norman Lowe 24 Lepidopteran Crossword No. 12 Nonconformist 25 Communications & links 26 GMS sponsors 27 Editorial – Norman Lowe Welcome to the final Garden Moth Scheme Quarterly Report of 2019. We have a bumper Christmas edition for you this time with a good variety of articles from lots of different contributors. Of course these always represent our contributors’ personal views, with which you might agree or disagree. And if so, let’s hear from you! We start as always with Evan’s roundup of the quarter’s results comparing moth catches with weather conditions as they occurred throughout the period. This time he has chosen to compare Ireland and Scotland, the northern and western extremities of our study area. They are also our two largest “regions” (OK I know they (and Wales) don’t like to be called regions but I can’t think of a better alternative) and the ones with the largest numbers of vice counties with no GMS recorders. I’ll return to this theme in my Tailpiece. Evan has also focused on the Setaceous Hebrew Character, a species that seems to vary possibly more than any other from place to place. -

Catalogue2013 Web.Pdf
bwfp British Wild Flower Plants www.wildflowers.co.uk Plants for Trade Plants for Home Specialist Species Wildflower Seed Green Roof Plants Over 350 species Scan here to of British native buy online plants 25th Anniversary Year Finding Us British Wild Flower Plants Burlingham Gardens 31 Main Road North Burlingham Norfolk NR13 4TA Phone / Fax: (01603) 716615 Email: [email protected] Website: http://www.wildflowers.co.uk Twitter: @WildflowersUK Nursery Opening Times Monday to Thursday: 10.00am - 4.00pm Friday: 10.00am - 2.30pm Please note that we are no longer open at weekends or Bank Holidays. Catalogue Contents Contact & Contents Page 02 About Us Page 03 Mixed Trays Pages 04-05 Reed Beds Page 06 Green Roofs Page 07 Wildflower Seeds Page 08 Planting Guide Pages 09-10 Attracting Wildlife Page 11 Rabbit-Proof Plants Page 12 List of Plants Pages 13-50 Scientific Name Look Up Pages 51-58 Terms & Conditions Page 59 www.wildflowers.co.uk 2 Tel/Fax:(01603)716615 About Us Welcome.... About Our Plants We are a family-run nursery, situated in Norfolk on a Our species are available most of the year in: six acre site. We currently stock over 350 species of 3 native plants and supply to all sectors of the industry Plugs: Young plants in 55cm cells with good rootstock. on a trade and retail basis. We are the largest grower of native plants in the UK and possibly Europe. Provenance Our species are drawn from either our own seed collections or from known provenance native sources. We comply with the Flora Locale Code of Practice. -

Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2
Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) by Boris C. Kondratieff, Paul A. Opler, Matthew C. Garhart, and Jason P. Schmidt C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 March 15, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration (top to bottom): Widow Skimmer (Libellula luctuosa) [photo ©Robert Behrstock], Stonefly (Perlesta species) [photo © David H. Funk, White- lined Sphinx (Hyles lineata) [photo © Matthew C. Garhart] ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences, Colorado State University, Fort Collins, Colorado 80523 Copyrighted 2004 Table of Contents EXECUTIVE SUMMARY……………………………………………………………………………….…1 INTRODUCTION…………………………………………..…………………………………………….…3 OBJECTIVE………………………………………………………………………………………….………5 Site Descriptions………………………………………….. METHODS AND MATERIALS…………………………………………………………………………….5 RESULTS AND DISCUSSION………………………………………………………………………..…...11 Dragonflies………………………………………………………………………………….……..11 -

Larval Protein Quality of Six Species of Lepidoptera (Saturniidaeo Sphingidae, Noctuidae)
Larval Protein Quality of Six Species of Lepidoptera (Saturniidaeo Sphingidae, Noctuidae) STEPHEN V. LANDRY,I GENE R. DEFOLIART,IANP MILTON L. SUNDE, University of Wisconsin, Madison, Wisconsin 53706 J. Econ. Entomol. 79; 600-604 (I98€) ABSTRACT Six lepidopteran species representing three families were evaluated for their potential use as protein supplements for poultry. Proximate and amino acid analyses were conducted on larval powders of each species. Larvae ranged from 49.4 to 58.1% crude protein on a dry-weight basis. Amino acid analysis indicated deficiencies in arginine, me- thionine, cysteine, and possibly lysine, when larvae are used in chick rations. In a chick- feeding trial with three of the species, however, these deffciencies were not substantiated: the average weight gained by chicks fed the lepidopteran-supplemented dlet did not differ significantly from that of.chicks fed a conventional corn/soybean control diet. LnprpoptBne ARE among the many species of in- by feeding trials on poultry (Ichhponani and Ma- sects that have played an important role in nutri- Iek 1971, Wijayasinghe and Rajaguru 1977). tion, especially in areas where human and domes- To determine and compare the protein quality tic animal populations are subject to chronic protein of a wider assortment of lepidopterous larvae, we deficiency (e.g., Bodenheimer 1951, Quin 1959, conducted proximate and amino acid analyses on Ruddle 1973, Conconi and Bourges 1977, Malaise larvae of six species representing three families. and Parent 1980, Conconi et al. 1984). Conconi et These included the cecropia moth, Hgalophora ce- al. (f9Sa), for example, listed 12 species in 8 fam- cropia (L.), and the promethea moth, Callosamia ilies that are gathered and consumed in Mexico. -

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -

Species Assessment for Jair Underwing
Species Status Assessment Class: Lepidoptera Family: Noctuidae Scientific Name: Catocala jair Common Name: Jair underwing Species synopsis: Two subspecies of Catocala exist-- Catocala jair and Catocala jair ssp2. Both occur in New York. Subspecies 2 has seldom been correctly identified leading to false statements that the species is strictly Floridian. Nearly all literature on the species neglects the widespread "subspecies 2." Cromartie and Schweitzer (1997) had it correct. Sargent (1976) discussed and illustrated the taxon but was undecided as to whether it was C. jair. It has also been called C. amica form or variety nerissa and one Syntype of that arguably valid taxon is jair and another is lineella. The latter should be chosen as a Lectotype to preserve the long standing use of jair for this species. Both D.F. Schweitzer and L.F. Gall have determined that subspecies 2 and typical jair are conspecific. The unnamed taxon should be named but there is little chance it is a separate species (NatureServe 2012). I. Status a. Current and Legal Protected Status i. Federal ____ Not Listed____________________ Candidate? _____No______ ii. New York ____Not Listed; SGCN_____ ___________________________________ b. Natural Heritage Program Rank i. Global _____G4?___________________________________________________________ ii. New York ______SNR_________ ________ Tracked by NYNHP? ____Yes____ Other Rank: None 1 Status Discussion: The long standing G4 rank needs to be re-evaluated. New Jersey, Florida, and Texas would probably drive the global rank. The species is still locally common on Long Island, but total range in New York is only a very small portion of Suffolk County (NatureServe 2012). II. Abundance and Distribution Trends a. -

Garden Moth Scheme Report 2016
Garden Moth Scheme Report 2016 Heather Young – April 2017 1 GMS Report 2016 CONTENTS PAGE Introduction 2 Top 30 species 2016 3 Population trends (?) of our commonest garden moths 5 Autumn Moths 12 Winter GMS 2016-17 14 Antler Moth infestations 16 GMS Annual Conference 2017 19 GMS Sponsors 20 Links & Acknowledgements 21 Cover photograph: Fan-foot (R. Young) Introduction The Garden Moth Scheme (GMS) welcomes participants from all parts of the United Kingdom and Ireland, and in 2016 received 341 completed recording forms, slightly fewer than last year (355). The scheme is divided into 12 regions, monitoring 233 species of moth in every part of the UK and Ireland (the ‘Core Species’), along with a variable number of ‘Regional Species’. For each of the last seven years, we have had records from over 300 sites across the UK and Ireland, and later in the report there are a series of charts representing the population trends (or fluctuations) of our most abundant species over this period. The database has records dating back to 2003 when the scheme began in the West Midlands, and now contains over 1 ¼ million records, providing a very valuable resource to researchers. Scientists and statisticians from Birmingham and Manchester Universities are amongst those interested in using our data, as well as the ongoing research being undertaken by the GMS’s own John Wilson. There is an interesting follow-up article by Evan Lynn on the Quarter 4 GMS newsletter piece by Duncan Brown on Antler Moth infestations, and a report on the very successful 2017 Annual Conference in Apperley Village Hall, near Tewkesbury. -

Immature Stages of the Marbled Underwing, Catocala Marmorata (Noctuidae)
JOURNAL OF THE LEPIDOPTERISTS' SOCIETY Volume 54 2000 Number 4 Journal of the Lepidopterists' Society 54(4), 2000,107- 110 IMMATURE STAGES OF THE MARBLED UNDERWING, CATOCALA MARMORATA (NOCTUIDAE) JOHN W PEACOCK 185 Benzler Lust Road, Marion, Ohio 43302, USA AND LAWRE:-JCE F GALL Entomology Division, Peabody Museum of Natural History, Yale University, New Haven, Connecticut 06520, USA ABSTRACT. The immature stages of C. marmorata are described and illustrated for the first time, along with biological and foodplant notes. Additional key words: underwing moths, Indiana, life history, Populus heterophylla. The Marbled Underwing, Catocala marrrwrata Ed REARING NOTES wards 1864, is generally an uncommon species whose present center of distribution is the central and south Ova were secured from a worn female C. rnarmorata central United States east of the Mississippi River collected at a baited tree at 2300 CST on 11 September (Fig. 1d). Historically, the range of C. marmorata ex 1994, in Point Twp. , Posey Co., Indiana. The habitat is tended somewhat farther to the north, as far as south mesic lowland flatwoods, with internal swamps of two ern New England (open circles in Fig. Id; see Holland types: (1) buttonbush (Cephalanthus occidentalis L.) 1903, Barnes & McDunnough 1918, Sargent 1976), (Rubiaceae), cypress (Taxodium distichum L. but the species has not been recorded from these lo (Richaud)) (Taxodiaceae), and swamp cottonwood calities in the past 50 years, and the reasons for its ap (Populus heterophylla L.); and (2) overcup oak (Quer parent range contraction remain unknown. cus lyrata Walt.) (Fagaceae) and swamp cottonwood. We are not aware of any previously published infor The female was confined in a large grocery bag (17.8 X mation on the early stages or larval foodplant(s) for C. -
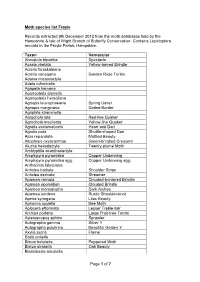
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Check List of Noctuid Moths (Lepidoptera: Noctuidae And
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (2), стор. 87–97, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (2), pp. 87–97, 2016 ARTICLE UDC 595.786 CHECK LIST OF NOCTUID MOTHS (LEPIDOPTERA: NOCTUIDAE AND EREBIDAE EXCLUDING LYMANTRIINAE AND ARCTIINAE) FROM THE SAUR MOUNTAINS (EAST KAZAKHSTAN AND NORTH-EAST CHINA) A.V. Volynkin1, 2, S.V. Titov3, M. Černila4 1 Altai State University, South Siberian Botanical Garden, Lenina pr. 61, Barnaul, 656049, Russia. E-mail: [email protected] 2 Tomsk State University, Laboratory of Biodiversity and Ecology, Lenina pr. 36, 634050, Tomsk, Russia 3 The Research Centre for Environmental ‘Monitoring’, S. Toraighyrov Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan. E-mail: [email protected] 4 The Slovenian Museum of Natural History, Prešernova 20, SI-1001, Ljubljana, Slovenia. E-mail: [email protected] The paper contains data on the fauna of the Lepidoptera families Erebidae (excluding subfamilies Lymantriinae and Arctiinae) and Noctuidae of the Saur Mountains (East Kazakhstan). The check list includes 216 species. The map of collecting localities is presented. Key words: Lepidoptera, Noctuidae, Erebidae, Asia, Kazakhstan, Saur, fauna. INTRODUCTION The fauna of noctuoid moths (the families Erebidae and Noctuidae) of Kazakhstan is still poorly studied. Only the fauna of West Kazakhstan has been studied satisfactorily (Gorbunov 2011). On the faunas of other parts of the country, only fragmentary data are published (Lederer, 1853; 1855; Aibasov & Zhdanko 1982; Hacker & Peks 1990; Lehmann et al. 1998; Benedek & Bálint 2009; 2013; Korb 2013). In contrast to the West Kazakhstan, the fauna of noctuid moths of East Kazakhstan was studied inadequately.