Immunity in Protochordates: the Tunicate Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chordata, Tunicata, Thaliacea, Doliolida) from East Coast of Peninsular Malaysia), with an Updated Worldwide Distribution
Journal of Sustainability Science and Management ISSN: 1823-8556 Volume 13 Number 5, 2018 © Penerbit UMT TAXONOMIC REVISION OF THE FAMILY DOLIOLIDAE BRONN, 1862 (CHORDATA, TUNICATA, THALIACEA, DOLIOLIDA) FROM EAST COAST OF PENINSULAR MALAYSIA), WITH AN UPDATED WORLDWIDE DISTRIBUTION NUR ‘ALIAH BINTI ADAM1 AND NURUL HUDA AHMAD ISHAK*1, 2 1School of Marine and Environmental Sciences, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia 2Institute of Oceanography and Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia *Corresponding author: [email protected] Abstract: The marine pelagic tunicate from the family of Doliolidae Bronn, 1862 in the coastal waters of Terengganu was studied for the first time, hereby presented in this paper. The distribution was analysed from 18 sampling stations alongside the Terengganu waters; including Pulau Bidong, Pulau Yu and Pulau Kapas. Samples were collected from April to July 2016 using 200µm Bongo net; towed vertically from a stationary vessel; and were preserved in a 5% buffered formaldehyde. Five species discovered in this family were identified as new records in Malaysian waters:Doliolum denticulatum Quoy and Gaimard, 1834, Doliolum nationalis Borgert, 1894, Dolioletta gegenbauri Uljanin, 1884, Doliolina mulleri Krohn, 1852 and Dolioloides rarum Grobben, 1882. A comprehensive review of the species description, diagnosis and a key to the phorozooid from the recorded species is herewith provided. We also deliver a detailed map of current and known worldwide occurrence of these five species, and thus consequently update the biodiversity of Malaysian fauna. KEYWORDS: Doliolid, pelagic tunicates, South China Sea, Terengganu, taxonomy, biogeography Introduction have the most complex life cycle compared to any of the pelagic tunicates; consisting of no lesser Pelagic tunicates are large transparent animals than six different and successive morphological that measure up to 25cm (Lavaniegos & Ohman, stages (Godeaux et al., 1998; Paffenhöfer & 2003). -

Colonial Tunicates: Species Guide
SPECIES IN DEPTH Colonial Tunicates Colonial Tunicates Tunicates are small marine filter feeder animals that have an inhalant siphon, which takes in water, and an exhalant siphon that expels water once it has trapped food particles. Tunicates get their name from the tough, nonliving tunic formed from a cellulose-like material of carbohydrates and proteins that surrounds their bodies. Their other name, sea squirts, comes from the fact that many species will shoot LambertGretchen water out of their bodies when disturbed. Massively lobate colony of Didemnum sp. A growing on a rope in Sausalito, in San Francisco Bay. A colony of tunicates is comprised of many tiny sea squirts called zooids. These INVASIVE SEA SQUIRTS individuals are arranged in groups called systems, which form interconnected Star sea squirts (Botryllus schlosseri) are so named because colonies. Systems of these filter feeders the systems arrange themselves in a star. Zooids are shaped share a common area for expelling water like ovals or teardrops and then group together in small instead of having individual excurrent circles of about 20 individuals. This species occurs in a wide siphons. Individuals and systems are all variety of colors: orange, yellow, red, white, purple, grayish encased in a matrix that is often clear and green, or black. The larvae each have eight papillae, or fleshy full of blood vessels. All ascidian tunicates projections that help them attach to a substrate. have a tadpole-like larva that swims for Chain sea squirts (Botryloides violaceus) have elongated, less than a day before attaching itself to circular systems. Each system can have dozens of zooids. -

Ascidian Cannibalism Correlates with Larval Behavior and Adult Distribution
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1988 Elsevier Ltd. The final published version of this manuscript is available at http://www.sciencedirect.com/science/journal/00220981 and may be cited as: Young, C. M. (1988). Ascidian cannibalism correlates with larval behavior and adult distribution. Journal of Experimental Marine Biology and Ecology, 117(1), 9-26. doi:10.1016/0022-0981(88)90068-8 J. Exp. Mar. Bioi. £Col., 1988, Vol. 117, pp. 9-26 9 Elsevier JEM 01042 Ascidian cannibalism correlates with larval behavior and adult distribution Craig M. Young Department ofLarval Ecology. Harbor Branch Oceanographic Institution, Fort Pierce, Florida. U.S.A. (Received 24 March 1987; revision received 9 December 1987; accepted 22 December 1987) Abstract: In the San Juan Islands, Washington, solitary ascidians .that occur in dense monospecific aggregations demonstrate gregarious settlement as larvae, whereas species that occur as isolated individuals do not. All gregarious species reject their own eggs and larvae as food, but nongregarious species consume conspecific eggs and larvae. Moreover, the rejection mechanism is species-specific in some cases. Correla tion analysis suggests that species specificity of the rejection response has a basis in siphon diameter, egg density, and larval size, but not in number of oral tentacles, or tentacle branching. One strongly cannibalistic species, Corella inflata Huntsman, avoids consuming its own eggs and newly released tadpoles by a unique brooding mechanism that involves floating eggs, negative geotaxis after hatching, and adult orientation. Key words: Ascidian; Cannibalism; Distribution; Larva; Settlement behavior INTRODUCTION Many sessile marine invertebrates, including filter-feeders such as mussels, oysters, barnacles and ascidians, occur in discrete, dense aggregations. -
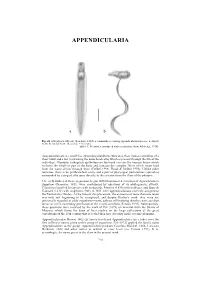
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Ascidiacea, Phlebobranchia, Corellidae) in the Southern Hemisphere with Description of a New Species
Zootaxa 3702 (2): 135–149 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3702.2.3 http://zoobank.org/urn:lsid:zoobank.org:pub:E972F88B-7981-4F38-803D-8F4F92FE6A37 The genus Corella (Ascidiacea, Phlebobranchia, Corellidae) in the Southern Hemisphere with description of a new species FRANÇOISE MONNIOT Muséum national d’Histoire naturelle, 57 rue Cuvier Fr 75231 Paris cedex 05, France.E-mail : [email protected] Abstract In the Southern Hemisphere the species attributed to Corella eumyota, Traustedt, 1882 are likely more varied than previously expected. This ascidian species was described from specimens collected at Valparaiso (Chile). Until now it was considered as a widely distributed species in the southern hemisphere. New collections from Chile and the Antarctic area have allowed to separate two species and re-establish Corella antarctica Sluiter, 1905 as a valid species (Alurralde 2013).A morphological re- examination of many specimens from the MNHN collections and especially recent surveys as CEAMARC and REVOLTA confirms that Antarctic specimens from the Antarctic Peninsula and Terre Adélie obviously differ from sub-Antarctic material more varied than previously estimated. On the other hand, C. eumyota invasive in Europe (Lambert 2004) has been shown to be the same as specimens from Chile, New Zealand and other sub-Antarctic regions. The present morphological study compares Corella from different regions and describes a new species Corella brewinae n. sp that is found living mixed with C. eumyota populations. Key words: Ascidians, Corellidae, Antarctic, Sub-Antarctic, new species Introduction The genus Corella was created by Hancock (1870) for Ascidia parallelogramma Müller, 1776. -

Phlebobranchia of CTAW
PHLEBOBRANCHIA PHLEBOBRANCHIA The suborder Phlebobranchia (order Enterogona) is characterised by having unpaired gonads present only on the same side of the body as the gut. As in Stolidobranchia, the body is not divided into different sections (such as thorax, abdomen and posterior abdomen) as the gut is folded up in the parietal body wall outside the pharynx and the large branchial sac occupies the whole length of the body. Usually the branchial sac (which is flat, without folds) has internal longitudinal vessels (although only vestiges remain in Agneziidae). Epicardial sacs do not persist in adults as they do in Aplousobranchia, although excretory vesicles (nephrocytes) embedded in the body wall over the gut are known to originate from the embryonic epicardium in Ascidiidae and Corellidae. Most phlebobranchs are solitary. However, Plurellidae Kott, 1973 includes both solitary and colonial forms, and Perophoridae Giard, 1872 are all colonial. Replication in Perophoridae is from ectodermal epithelium (rather than endodermal or mesodermal tissue the mesodermal tissue of the vascular stolon (rather than the endodermal tissue as in most as in Aplousobranchia). The process of replication has not been investigated in Plurellidae. Phlebobranch taxa occurring in Australia are documented in Kott (1985). Family level taxa are characterised principally by the size and form of the branchial sac including the number of branchial vessels and form of the stigmata; the form, size and position of the gonads; and the habit (colonial or solitary) of the taxon. Berrill (1950) has discussed problems in assessing the phylogeny of Perophoridae. References Berrill, N.J. (1950). The Tunicata. Ray Soc. Publs 133: 1–354 Giard, A.M. -

Eudistoma (Ascidiacea: Polycitoridae) from Tropical Brazil
ZOOLOGIA 31 (2): 195–208, April, 2014 http://dx.doi.org/10.1590/S1984-46702014000200011 Eudistoma (Ascidiacea: Polycitoridae) from tropical Brazil Livia de Moura Oliveira1, Gustavo Antunes Gamba1 & Rosana Moreira da Rocha1,2 1 Programa de Pós-graduação em Zoologia, Departamento de Zoologia, Universidade Federal do Paraná. Caixa Postal 19020, 81531-980 Curitiba, PR, Brazil. 2 Corresponding author: E-mail: [email protected] ABSTRACT. We studied material in collections from coastal intertidal and subtidal tropical waters of the Brazilian states of Paraíba, Pernambuco, Alagoas, Bahia, and Espírito Santo. We identified seven species of Eudistoma, of which two are new to science. Eudistoma alvearium sp. nov. colonies have fecal pellets around each zooid and zooids are 6-8 mm long with seven straight and parallel pyloric tubules; the larval trunk is 0.6 mm long with three adhesive papillae and ten ampullae. Eudistoma versicolor sp. nov. colonies are cushion-shaped, variable in color (blue, purple, brown, light green, gray or white) and zooids have six straight and parallel pyloric tubules; the larval trunk is 0.8 mm long with three adhesive papillae and six ampules. Three species – E. carolinense Van Name, 1945, E. recifense Millar, 1977, and E. vannamei Millar, 1977 – are known from northeastern Brazil. The identification of two additional species will require confirmation. We also propose a synonymy for E. carolinense with E. repens Millar, 1977, also previously described in Brazil. KEY WORDS. Atlantic; colonial ascidians; new species; taxonomy. Eudistoma Caullery, 1909 is the most species-rich genus and comment on the implications of species richness for the in Polycitoridae, with 124 valid species found in tropical and distribution of Eudistoma. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Preliminary Mass-Balance Food Web Model of the Eastern Chukchi Sea
NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center December 2013 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Whitehouse, G. A. 2013. A preliminary mass-balance food web model of the eastern Chukchi Sea. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-262, 162 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse1,2 1Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle WA 98115 2Joint Institute for the Study of the Atmosphere and Ocean University of Washington Box 354925 Seattle WA 98195 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Penny. S. Pritzker, Secretary National Oceanic and Atmospheric Administration Kathryn D. -

Halocynthia Roretzi
Sekigami et al. Zoological Letters (2017) 3:17 DOI 10.1186/s40851-017-0078-3 RESEARCH ARTICLE Open Access Hox gene cluster of the ascidian, Halocynthia roretzi, reveals multiple ancient steps of cluster disintegration during ascidian evolution Yuka Sekigami1, Takuya Kobayashi1, Ai Omi1, Koki Nishitsuji2, Tetsuro Ikuta1, Asao Fujiyama3, Noriyuki Satoh2 and Hidetoshi Saiga1* Abstract Background: Hox gene clusters with at least 13 paralog group (PG) members are common in vertebrate genomes and in that of amphioxus. Ascidians, which belong to the subphylum Tunicata (Urochordata), are phylogenetically positioned between vertebrates and amphioxus, and traditionally divided into two groups: the Pleurogona and the Enterogona. An enterogonan ascidian, Ciona intestinalis (Ci), possesses nine Hox genes localized on two chromosomes; thus, the Hox gene cluster is disintegrated. We investigated the Hox gene cluster of a pleurogonan ascidian, Halocynthia roretzi (Hr) to investigate whether Hox gene cluster disintegration is common among ascidians, and if so, how such disintegration occurred during ascidian or tunicate evolution. Results: Our phylogenetic analysis reveals that the Hr Hox gene complement comprises nine members, including one with a relatively divergent Hox homeodomain sequence. Eight of nine Hr Hox genes were orthologous to Ci-Hox1, 2, 3, 4, 5, 10, 12 and 13. Following the phylogenetic classification into 13 PGs, we designated Hr Hox genes as Hox1, 2, 3, 4, 5, 10, 11/12/13.a, 11/12/13.b and HoxX. To address the chromosomal arrangement of the nine Hox genes, we performed two-color chromosomal fluorescent in situ hybridization, which revealed that the nine Hox genes are localized on a single chromosome in Hr, distinct from their arrangement in Ci. -

Aliens in Egyptian Waters. a Checklist of Ascidians of the Suez Canal and the Adjacent Mediterranean Waters
Egyptian Journal of Aquatic Research (2016) xxx, xxx–xxx HOSTED BY National Institute of Oceanography and Fisheries Egyptian Journal of Aquatic Research http://ees.elsevier.com/ejar www.sciencedirect.com FULL LENGTH ARTICLE Aliens in Egyptian waters. A checklist of ascidians of the Suez Canal and the adjacent Mediterranean waters Y. Halim a, M. Abdel Messeih b,* a Oceanography Department, Faculty of Science, Alexandria, Egypt b National Institute of Oceanography and Fisheries, Alexandria, Egypt Received 3 April 2016; revised 21 August 2016; accepted 22 August 2016 KEYWORDS Abstract Checklists of the alien ascidian fauna of Egyptian waters are provided covering the Suez Ascidians; Canal, the adjacent Mediterranean waters and the Gulf of Suez. Enrichment in ascidian species of Mediterranean Sea; the Suez Canal seems to have been on the increase since 1927. The distinctly uneven distribution Erythrean non-indigenous pattern in the Canal appears to be directly related to the ship traffic system. species; Earlier reports on alien ascidian species in the Mediterranean are compared and discussed. Of 65 Suez Canal; species recorded from the Mediterranean waters of Egypt in all, four are Erythrean migrants and Polyclinum constellatum four potentially so. Polyclinum constellatum Savigny, 1816 is a new record for the Mediterranean Sea. Ó 2016 National Institute of Oceanography and Fisheries. Hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Introduction 2005 and 2014 to deal with this issue and with other related problems. Ascidians are receiving more and more attention because of Based on an analysis of the literature and on the on-line the invasive ability of some species and the severe damage World Register of Marine Species (www.marinespecies.org/), caused to aquaculture (reviewed in a special issue of Aquatic Shenkar and Swalla (2011) assembled 2815 described ascidian Invasions, January 2009: http://aquatic invasions.net/2009/in- species. -

1471-2148-9-187.Pdf
BMC Evolutionary Biology BioMed Central Research article Open Access An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models Georgia Tsagkogeorga1,2, Xavier Turon3, Russell R Hopcroft4, Marie- Ka Tilak1,2, Tamar Feldstein5, Noa Shenkar5,6, Yossi Loya5, Dorothée Huchon5, Emmanuel JP Douzery1,2 and Frédéric Delsuc*1,2 Address: 1Université Montpellier 2, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 2CNRS, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 3Centre d'Estudis Avançats de Blanes (CEAB, CSIC), Accés Cala S. Francesc 14, 17300 Blanes (Girona), Spain, 4Institute of Marine Science, University of Alaska Fairbanks, Fairbanks, Alaska, USA, 5Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, 69978, Israel and 6Department of Biology, University of Washington, Seattle WA 98195, USA Email: Georgia Tsagkogeorga - [email protected]; Xavier Turon - [email protected]; Russell R Hopcroft - [email protected]; Marie-Ka Tilak - [email protected]; Tamar Feldstein - [email protected]; Noa Shenkar - [email protected]; Yossi Loya - [email protected]; Dorothée Huchon - [email protected]; Emmanuel JP Douzery - [email protected]; Frédéric Delsuc* - [email protected] * Corresponding author Published: 5 August 2009 Received: 16 October 2008 Accepted: 5 August 2009 BMC Evolutionary Biology 2009, 9:187 doi:10.1186/1471-2148-9-187 This article is available from: http://www.biomedcentral.com/1471-2148/9/187 © 2009 Tsagkogeorga et al; licensee BioMed Central Ltd.