Dctd Programs and Initiatives (2013-2017) Biometric Research Program 46
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Hypotensive Effect of Activated Apelin Receptor Is Correlated with Β
This is the accepted (postprint) version of the following article: Besserer-Offroy É, et al. (2018), Pharmacol Res. doi: 10.1016/j.phrs.2018.02.032, which has been accepted and published in its final form at https://www.sciencedirect.com/science/article/pii/S1043661817313804 The hypotensive effect of activated apelin receptor is correlated with β-arrestin recruitment Élie Besserer-Offroya,c,ORCID ID, Patrick Bérubéa,c, Jérôme Côtéa,c, Alexandre Murzaa,c, Jean-Michel Longpréa,c, Robert Dumainea, Olivier Lesurb,c, Mannix Auger-Messierb,ORCID ID, Richard Leduca,c,ORCID ID, Éric Marsaulta,c,*,ORCID ID, Philippe Sarreta,c* Affiliations a Department of Pharmacology-Physiology, Faculty of Medicine and Health Sciences, Université de Sherbrooke, Sherbrooke, Québec, CANADA J1H 5N4 b Department of Medicine, Faculty of Medicine and Health Sciences, Université de Sherbrooke, Sherbrooke, Québec, CANADA J1H 5N4 c Institut de pharmacologie de Sherbrooke, Université de Sherbrooke, Sherbrooke, Québec, CANADA J1H 5N4 e-mail addresses [email protected] (ÉBO); [email protected] (PB); [email protected] (JC); [email protected] (AM); Jean- [email protected] (JML); [email protected] (RD); [email protected] (OL); [email protected] (MAM); [email protected] (RL); [email protected] (EM); [email protected] (PS) © 2018. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ This is the accepted (postprint) version of the following article: Besserer-Offroy É, et al. (2018), Pharmacol Res. doi: 10.1016/j.phrs.2018.02.032, which has been accepted and published in its final form at https://www.sciencedirect.com/science/article/pii/S1043661817313804 Corresponding Authors *To whom correspondence should be addressed: Philippe Sarret, Ph.D.; [email protected]; Tel. -

Oncolytic Viruses PANVAC and Pelareorep As Treatment for Metastatic Breast Cancer
Oncolytic Viruses PANVAC and Pelareorep as Treatment for Metastatic Breast Cancer Willie Mieke Iwema Rijksuniversiteit Groningen S2673622 BSc Thesis Life science & Technology July 5, 2019 Department of Medical Microbiology: Molecular Virology D. Bhatt PhD candidate Prof. dr. C.A.H.H. Daemen Table of content 1. Introduction ................................................................................................................................ 4 2. PANVAC ....................................................................................................................................... 7 2.1 Vaccinia virus mechanism of action .............................................................................................. 7 2.2 Activation of the host immune system .......................................................................................... 8 2.3 PANVAC in advanced carcinomas ................................................................................................. 9 2.4 PANVAC and docetaxel in metastatic breast cancer ................................................................... 11 3. Pelareorep ................................................................................................................................. 13 3.1 Reovirus mechanism of action .................................................................................................... 13 3.2 Activation of the host immune system ........................................................................................ 14 3.3 Pelareorep -

How I Treat Myelofibrosis
From www.bloodjournal.org by guest on October 7, 2014. For personal use only. Prepublished online September 16, 2014; doi:10.1182/blood-2014-07-575373 How I treat myelofibrosis Francisco Cervantes Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Advance online articles have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Advance online articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Advance online articles must include digital object identifier (DOIs) and date of initial publication. Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From www.bloodjournal.org by guest on October 7, 2014. For personal use only. Blood First Edition Paper, prepublished online September 16, 2014; DOI 10.1182/blood-2014-07-575373 How I treat myelofibrosis By Francisco Cervantes, MD, PhD, Hematology Department, Hospital Clínic, IDIBAPS, University of Barcelona, Barcelona, Spain Correspondence: Francisco Cervantes, MD, Hematology Department, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain. Phone: +34 932275428. -

PARP1 Trapping by PARP Inhibitors Drives Cytotoxicity Both in Cancer Cells and Healthy Bone Marrow
Author Manuscript Published OnlineFirst on November 14, 2018; DOI: 10.1158/1541-7786.MCR-18-0138 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 Title: PARP1 trapping by PARP inhibitors drives cytotoxicity both in cancer 2 cells and healthy bone marrow 3 4 Authors: Todd A. Hopkins, William B. Ainsworth, Paul A. Ellis, Cherrie K. 5 Donawho, Enrico L. DiGiammarino, Sanjay C. Panchal, Vivek C. 6 Abraham, Mikkel A. Algire, Yan Shi, Amanda M. Olson, Eric F. Johnson, 7 Julie L. Wilsbacher, David Maag* 8 9 Author affiliations: AbbVie, Inc., North Chicago, Illinois, USA 10 Running title: PARP trapping: impact on in vivo activity of PARP inhibitors 11 Keywords: PARP, DNA damage, veliparib,talazoparib, rucaparib, A-934935 12 Financial support: This work was financially supported by AbbVie, Inc. 13 Corresponding author: *David Maag, Oncology Development, AbbVie, Inc., 1 N. Waukegan 14 Road, North Chicago, Illinois, USA 60064. Phone: 847-937-3969; E- 15 mail: [email protected]. 16 Disclosure: All authors are employees of AbbVie. The design, study conduct, and 17 financial support for this research were provided by AbbVie. AbbVie 18 participated in the interpretation of data, review, and approval of the 19 publication. 20 Word count: 5163/5000 21 Total number of figures and tables: 12 (6 plus 6 supplemental) 22 1 Downloaded from mcr.aacrjournals.org on September 26, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on November 14, 2018; DOI: 10.1158/1541-7786.MCR-18-0138 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -
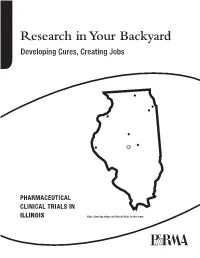
Research in Your Backyard Developing Cures, Creating Jobs
Research in Your Backyard Developing Cures, Creating Jobs PHARMACEUTICAL CLINICAL TRIALS IN ILLINOIS Dots show locations of clinical trials in the state. Executive Summary This report shows that biopharmaceutical research com- Quite often, biopharmaceutical companies hire local panies continue to be vitally important to the economy research institutions to conduct the tests and in Illinois, and patient health in Illinois, despite the recession. they help to bolster local economies in communities all over the state, including Chicago, Decatur, Joliet, Peoria, At a time when the state still faces significant economic Quincy, Rock Island, Rockford and Springfield. challenges, biopharmaceutical research companies are conducting or have conducted more than 4,300 clinical For patients, the trials offer another potential therapeutic trials of new medicines in collaboration with the state’s option. Clinical tests may provide a new avenue of care for clinical research centers, university medical schools and some chronic disease sufferers who are still searching for hospitals. Of the more than 4,300 clinical trials, 2,334 the medicines that are best for them. More than 470 of the target or have targeted the nation’s six most debilitating trials underway in Illinois are still recruiting patients. chronic diseases—asthma, cancer, diabetes, heart dis- ease, mental illnesses and stroke. Participants in clinical trials can: What are Clinical Trials? • Play an active role in their health care. • Gain access to new research treatments before they In the development of new medicines, clinical trials are are widely available. conducted to prove therapeutic safety and effectiveness and compile the evidence needed for the Food and Drug • Obtain expert medical care at leading health care Administration to approve treatments. -

Pembrolizumab in Combination with the Oncolytic Virus Pelareorep And
Published OnlineFirst November 6, 2019; DOI: 10.1158/1078-0432.CCR-19-2078 CLINICAL CANCER RESEARCH | CLINICAL TRIALS: IMMUNOTHERAPY Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study A C Devalingam Mahalingam1,2, Grey A. Wilkinson3, Kevin H. Eng4, Paul Fields5, Patrick Raber5, Jennifer L. Moseley2, Karol Cheetham3, Matt Coffey3, Gerard Nuovo6, Pawel Kalinski4, Bin Zhang1, Sukeshi Patel Arora2, and Christos Fountzilas4 ABSTRACT ◥ Background: Pelareorep is an intravenously delivered oncolytic achieved partial response for 17.4 months. Two additional patients reovirus that can induce a T-cell–inflamed phenotype in pancreatic achieved stable disease, lasting 9 and 4 months, respectively. ductal adenocarcinoma (PDAC). Tumor tissues from patients Treatment was well tolerated, with mostly grade 1 or 2 treat- treated with pelareorep have shown reovirus replication, T-cell ment-related adverse events, including flu-like symptoms. Viral infiltration, and upregulation of PD-L1. We hypothesized that replication was observed in on-treatment tumor biopsies. T-cell pelareorep in combination with pembrolizumab and chemotherapy receptor sequencing from peripheral blood revealed the creation of in patients with PDAC would be safe and effective. new T-cell clones during treatment. High peripheral clonality and Methods: A phase Ib single-arm study enrolled patients with changes in the expression of immune genes were observed in PDAC who progressed after first-line treatment. Patients received patients with clinical benefit. pelareorep, pembrolizumab, and either 5-fluorouracil, gemcitabine, Conclusions: Pelareorep and pembrolizumab added to che- or irinotecan until disease progression or unacceptable toxicity. motherapy did not add significant toxicity and showed encour- Study objectives included safety and dose-limiting toxicities, tumor aging efficacy. -

Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives
cancers Review Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives Shirin Hafezi 1 and Mohamed Rahmani 1,2,* 1 Research Institute of Medical & Health Sciences, University of Sharjah, P.O. Box 27272 Sharjah, United Arab Emirates; [email protected] 2 Department of Basic Medical Sciences, College of Medicine, University of Sharjah, P.O. Box 27272 Sharjah, United Arab Emirates * Correspondence: [email protected]; Tel.: +971-6505-7759 Simple Summary: Apoptosis, a programmed form of cell death, represents the main mechanism by which cells die. Such phenomenon is highly regulated by the BCL-2 family of proteins, which includes both pro-apoptotic and pro-survival proteins. The decision whether cells live or die is tightly controlled by a balance between these two classes of proteins. Notably, the pro-survival Bcl-2 proteins are frequently overexpressed in cancer cells dysregulating this balance in favor of survival and also rendering cells more resistant to therapeutic interventions. In this review, we outlined the most important steps in the development of targeting the BCL-2 survival proteins, which laid the ground for the discovery and the development of the selective BCL-2 inhibitor venetoclax as a therapeutic drug in hematological malignancies. The limitations and future directions are also discussed. Abstract: The major form of cell death in normal as well as malignant cells is apoptosis, which is a programmed process highly regulated by the BCL-2 family of proteins. This includes the antiapoptotic proteins (BCL-2, BCL-XL, MCL-1, BCLW, and BFL-1) and the proapoptotic proteins, which can be divided into two groups: the effectors (BAX, BAK, and BOK) and the BH3-only proteins Citation: Hafezi, S.; Rahmani, M. -

MET Or NRAS Amplification Is an Acquired Resistance Mechanism to the Third-Generation EGFR Inhibitor Naquotinib
www.nature.com/scientificreports OPEN MET or NRAS amplifcation is an acquired resistance mechanism to the third-generation EGFR inhibitor Received: 5 October 2017 Accepted: 16 January 2018 naquotinib Published: xx xx xxxx Kiichiro Ninomiya1, Kadoaki Ohashi1,2, Go Makimoto1, Shuta Tomida3, Hisao Higo1, Hiroe Kayatani1, Takashi Ninomiya1, Toshio Kubo4, Eiki Ichihara2, Katsuyuki Hotta5, Masahiro Tabata4, Yoshinobu Maeda1 & Katsuyuki Kiura2 As a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), osimeritnib is the standard treatment for patients with non-small cell lung cancer harboring the EGFR T790M mutation; however, acquired resistance inevitably develops. Therefore, a next-generation treatment strategy is warranted in the osimertinib era. We investigated the mechanism of resistance to a novel EGFR-TKI, naquotinib, with the goal of developing a novel treatment strategy. We established multiple naquotinib-resistant cell lines or osimertinib-resistant cells, two of which were derived from EGFR-TKI-naïve cells; the others were derived from geftinib- or afatinib-resistant cells harboring EGFR T790M. We comprehensively analyzed the RNA kinome sequence, but no universal gene alterations were detected in naquotinib-resistant cells. Neuroblastoma RAS viral oncogene homolog (NRAS) amplifcation was detected in naquotinib-resistant cells derived from geftinib-resistant cells. The combination therapy of MEK inhibitors and naquotinib exhibited a highly benefcial efect in resistant cells with NRAS amplifcation, but the combination of MEK inhibitors and osimertinib had limited efects on naquotinib-resistant cells. Moreover, the combination of MEK inhibitors and naquotinib inhibited the growth of osimertinib-resistant cells, while the combination of MEK inhibitors and osimertinib had little efect on osimertinib-resistant cells. -

New Contributions in Undergraduate Research
PSU McNair Scholars Online Journal Volume 11 Issue 1 Without Borders: Original Contributions Article 6 in Undergraduate Research 2017 Wings Outstretched: New Contributions in Undergraduate Research Follow this and additional works at: https://pdxscholar.library.pdx.edu/mcnair Let us know how access to this document benefits ou.y Recommended Citation (2017) "Wings Outstretched: New Contributions in Undergraduate Research," PSU McNair Scholars Online Journal: Vol. 11: Iss. 1, Article 6. https://doi.org/10.15760/mcnair.2017.01 This open access Full Issue is distributed under the terms of the Creative Commons Attribution-NonCommercial- ShareAlike 4.0 International License (CC BY-NC-SA 4.0). All documents in PDXScholar should meet accessibility standards. If we can make this document more accessible to you, contact our team. Portland State University McNair Research Journal 2017 Without Borders: Original Contributions in Undergraduate Research 2017 Ronald E. McNair Scholars Journal Portland State University 1 About the Program The Portland State University (PSU) Ronald E. McNair Scholars Program at Portland State University works with motivated and talented undergraduates who want to pursue PhDs. It introduces juniors and seniors who are first-generation and low income, and/or members of under-represented groups to academic research and to effective strategies for getting into and graduating from PhD programs. The McNair Scholars Program has academic-year activities and a full-time summer research internship. Scholars take academic and skills-building seminars and workshops during the year, and each scholar works closely with a faculty mentor on original research in the summer. Scholars present their research findings at the McNair Summer Symposium and at other conferences, and are encouraged to publish their papers in the McNair Journal and other scholarly publications. -

Daiichi Sankyo Group Value Report 2019
External Evaluations (as of June 30,2019) ™ Daiichi Sankyo Group Value Report 2019 Value Daiichi Sankyo Group MSCI Japan Empowering Women Select Index THE INCLUSION OF DAIICHI SANKYO CO.,LTD. IN ANY MSCI INDEX, AND THE USE OF MSCI LOGOS, TRADEMARKS, SERVICE MARKS OR INDEX NAMES HEREIN, DO NOT CONSTITUTE A SPONSORSHIP, ENDORSEMENT OR PROMOTION OF DAIICHI SANKYO CO.,LTD. BY MSCI OR ANY OF ITS AFFILIATES. THE MSCI INDEXES ARE THE EXCLUSIVE PROPERTY OF MSCI. MSCI AND THE MSCI INDEX NAMES AND LOGOS ARE TRADE- MARKS OR SERVICE MARKS OF MSCI OR ITS AFFILIATES. “Eruboshi” Certification Mark “Kurumin” Certification Mark Logo given to Certified Health and Productivity Management Organization (White500) This report uses FSC® certified paper, which indicates that the paper used to print this Paper report was produced from properly managed forests. 3-5-1, Nihonbashi-honcho, Chuo-ku, Tokyo 103-8426, Japan This report was printed using 100% Inks biodegradable printing inks from vegetable Corporate Communications Department oil. Daiichi Sankyo Group Tel: +81-3-6225-1126 CSR Department The waterless printing method used for this Value Report 2019 Tel: +81-3-6225-1067 Printing report minimized the use and release of harmful liquid wastes. https://www.daiichisankyo.com/ Printed in Japan 005_7045687911909.indd 1 2019/09/27 18:22:19 Introduction Our Mission The Core Values and Commitments serve as the criteria for business activities and In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter . decision-making used by executive officers and employees in working to fulfill Our Mission . This charter calls on us to fulfill our social responsibilities by acting with the highest ethical Our Corporate Slogan succinctly explains the spirit of Our Mission, Core Values and standards and a good social conscience appropriate for a company engaged in business Commitments. -

Targeted Therapies in Melanoma: Knowledge, Resistance And
ooggeenneessii iinn ss && rrcc aa MM CC uu tt ff aa Journal ofJournal of oo gg ll ee ee aa aa nn nn nn nn ee ee rr rr ss ss uu uu ii ii Colombino et al., J Carcinog Mutagen 2014, S4:S4 ss ss oo oo JJ JJ ISSN: 2157-2518 CarCarcinogenesiscinogenesis & Mutagenesis DOI: 10.4172/2157-2518.S4-004 Review Article Open Access Targeted Therapies in Melanoma: Knowledge, Resistance and Perspectives Maria Colombino1*, Maria Cristina Sini1, Amelia Lissia2, Antonio Cossu2, and Giuseppe Palmieri1 1Unit of Cancer Genetics, Institute of Biomolecular Chemistry (ICB), National Research Council (CNR), Italy 2University Hospital Health Unit - Azienda Ospedaliero Universitaria (AOU), Via Matteotti, 07100 Sassari, Italy *Corresponding author: Dr. Maria Colombino, Unit of Cancer Genetics, Institute of Biomolecular Chemistry (ICB), National Research Council (CNR), Traversa La Crucca, 3 - Baldinca Li Punti, 07100 Sassari, Italy, Tel. +39 079 2841239; Fax +39 079 2841299; E-mail: [email protected] Received date: Mar 18, 2014, Accepted date: May 25, 2014, Published date: May 31, 2014 Copyright: © 2014 Colombino M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Several molecular mechanisms appear to play a major role in melanoma genesis and progression. Current targeted therapies focus on contrasting the activation of RAS/RAF/MEK/ERK and, to a less extent, PI3K/AKT pathways. Development of inhibitors of key effectors (mainly, BRAF mutant and MEK) has significantly improved treatment of patients with advanced melanoma. -

AIFM2 Monoclonal Antibody (M13A), Clone 2C6
AIFM2 monoclonal antibody (M13A), clone 2C6 Catalog # : H00084883-M13A 規格 : [ 200 uL ] List All Specification Application Image Product Mouse monoclonal antibody raised against a partial recombinant Western Blot (Transfected lysate) Description: AIFM2. Immunogen: AIFM2 (AAH06121, 1 a.a. ~ 339 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Sequence: MGSQVSVESGALHVVIVGGGFGGIAAASQLQALNVPFMLVDMKDSFHHN VAALRASVETGFAKKTFISYSVTFKDNFRQGLVVGIDLKNQMVLLQGGE ALPFSHLILATGSTGPFPGKFNEVSSQQAAIQAYEDMVRQVQRSRFIVVV enlarge GGGSAGVEMAAEIKTEYPEKEVTLIHSQVALADKELLPSVRQEVKEILLRK Western Blot (Recombinant GVQLLLSERVSNLEELPLNEYREYIKVQTDKGTEVATNLVILCTGIKINSSA protein) YRKAFESRLASSGALRVNEHLQVEGHSNVYAIGDCADVRTPKMAYLAGL HANIAVANIVNSVKQRPLQAYKPGALTFLLSMGRNDGVG ELISA Host: Mouse Reactivity: Human Isotype: IgG1 Kappa Quality Control Antibody Reactive Against Recombinant Protein. Testing: Western Blot detection against Immunogen (62.92 KDa) . Storage Buffer: In ascites fluid Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Interspecies Mouse (90); Rat (90) Antigen Sequence: Datasheet: Download Applications Western Blot (Transfected lysate) Page 1 of 3 2021/6/18 Western Blot analysis of AIFM2 expression in transfected 293T cell line by AMID monoclonal antibody (M13A), clone 2C6. Lane 1: AIFM2 transfected lysate(40.5 KDa). Lane 2: Non-transfected lysate. Protocol Download Western Blot (Recombinant protein) Protocol Download ELISA Gene Information Entrez GeneID: 84883 GeneBank BC006121 Accession#: Protein AAH06121 Accession#: Gene Name: AIFM2 Gene Alias: AMID,PRG3,RP11-367H5.2 Gene apoptosis-inducing factor, mitochondrion-associated, 2 Description: Omim ID: 605159 Gene Ontology: Hyperlink Gene Summary: The protein encoded by this gene has significant homology to NADH oxidoreductases and the apoptosis-inducing factor PDCD8/AIF. Overexpression of this gene has been shown to induce apoptosis. The expression of this gene is found to be induced by tumor suppressor protein p53 in colon caner cells.