Appendix 1: Anaphylaxis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiating Between Anxiety, Syncope & Anaphylaxis
Differentiating between anxiety, syncope & anaphylaxis Dr. Réka Gustafson Medical Health Officer Vancouver Coastal Health Introduction Anaphylaxis is a rare but much feared side-effect of vaccination. Most vaccine providers will never see a case of true anaphylaxis due to vaccination, but need to be prepared to diagnose and respond to this medical emergency. Since anaphylaxis is so rare, most of us rely on guidelines to assist us in assessment and response. Due to the highly variable presentation, and absence of clinical trials, guidelines are by necessity often vague and very conservative. Guidelines are no substitute for good clinical judgment. Anaphylaxis Guidelines • “Anaphylaxis is a potentially life-threatening IgE mediated allergic reaction” – How many people die or have died from anaphylaxis after immunization? Can we predict who is likely to die from anaphylaxis? • “Anaphylaxis is one of the rarer events reported in the post-marketing surveillance” – How rare? Will I or my colleagues ever see a case? • “Changes develop over several minutes” – What is “several”? 1, 2, 10, 20 minutes? • “Even when there are mild symptoms initially, there is a potential for progression to a severe and even irreversible outcome” – Do I park my clinical judgment at the door? What do I look for in my clinical assessment? • “Fatalities during anaphylaxis usually result from delayed administration of epinephrine and from severe cardiac and respiratory complications. “ – What is delayed? How much time do I have? What is anaphylaxis? •an acute, potentially -

Appendix A: Potentially Inappropriate Prescriptions (Pips) for Older People (Modified from ‘STOPP/START 2’ O’Mahony Et Al 2014)
Appendix A: Potentially Inappropriate Prescriptions (PIPs) for older people (modified from ‘STOPP/START 2’ O’Mahony et al 2014) Consider holding (or deprescribing - consult with patient): 1. Any drug prescribed without an evidence-based clinical indication 2. Any drug prescribed beyond the recommended duration, where well-defined 3. Any duplicate drug class (optimise monotherapy) Avoid hazardous combinations e.g.: 1. The Triple Whammy: NSAID + ACE/ARB + diuretic in all ≥ 65 year olds (NHS Scotland 2015) 2. Sick Day Rules drugs: Metformin or ACEi/ARB or a diuretic or NSAID in ≥ 65 year olds presenting with dehydration and/or acute kidney injury (AKI) (NHS Scotland 2015) 3. Anticholinergic Burden (ACB): Any additional medicine with anticholinergic properties when already on an Anticholinergic/antimuscarinic (listed overleaf) in > 65 year olds (risk of falls, increased anticholinergic toxicity: confusion, agitation, acute glaucoma, urinary retention, constipation). The following are known to contribute to the ACB: Amantadine Antidepressants, tricyclic: Amitriptyline, Clomipramine, Dosulepin, Doxepin, Imipramine, Nortriptyline, Trimipramine and SSRIs: Fluoxetine, Paroxetine Antihistamines, first generation (sedating): Clemastine, Chlorphenamine, Cyproheptadine, Diphenhydramine/-hydrinate, Hydroxyzine, Promethazine; also Cetirizine, Loratidine Antipsychotics: especially Clozapine, Fluphenazine, Haloperidol, Olanzepine, and phenothiazines e.g. Prochlorperazine, Trifluoperazine Baclofen Carbamazepine Disopyramide Loperamide Oxcarbazepine Pethidine -

Chlorphenamine Maleate)
Package leaflet: Information for the patient Chlorphenamine 10 mg/ml Solution for Injection (Chlorphenamine Maleate) Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. − Keep this leaflet. You may need to read it again. − If you have any further questions, ask your doctor or nurse. − If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Chlorphenamine is and what it is used for 2. What you need to know before Chlorphenamine is given 3. How Chlorphenamine is given 4. Possible side effects 5. How to store Chlorphenamine 6. Contents of the pack and other information 1. What Chlorphenamine is and what it is used for Chlorphenamine 10 mg/ml Solution for Injection contains the active ingredient chlorphenamine maleate which is an antihistamine. Chlorphenamine is indicated in adults and children (aged 1 month to 18 years) for the treatment of acute allergic reactions. These medicines inhibit the release of histamine into the body that occurs during an allergic reaction. This product relieves some of the main symptoms of a severe allergic reaction. 2. What you need to know before Chlorphenamine is given You MUST NOT be given Chlorphenamine: if you are allergic to chlorphenamine maleate or any of the other ingredients of this medicine (listed in section 6) if you have had monoamine oxidase inhibitor (MAOI) antidepressive treatment within the past 14 days. Warnings and precautions Talk to your doctor or nurse before you are given this medicine if you: are being treated for an overactive thyroid or enlarged prostate gland have epilepsy, raised blood pressure within the eye or glaucoma, very high blood pressure, heart, liver, asthma or other chest diseases. -

FSI-D-16-00226R1 Title
Elsevier Editorial System(tm) for Forensic Science International Manuscript Draft Manuscript Number: FSI-D-16-00226R1 Title: An overview of Emerging and New Psychoactive Substances in the United Kingdom Article Type: Review Article Keywords: New Psychoactive Substances Psychostimulants Lefetamine Hallucinogens LSD Derivatives Benzodiazepines Corresponding Author: Prof. Simon Gibbons, Corresponding Author's Institution: UCL School of Pharmacy First Author: Simon Gibbons Order of Authors: Simon Gibbons; Shruti Beharry Abstract: The purpose of this review is to identify emerging or new psychoactive substances (NPS) by undertaking an online survey of the UK NPS market and to gather any data from online drug fora and published literature. Drugs from four main classes of NPS were identified: psychostimulants, dissociative anaesthetics, hallucinogens (phenylalkylamine-based and lysergamide-based materials) and finally benzodiazepines. For inclusion in the review the 'user reviews' on drugs fora were selected based on whether or not the particular NPS of interest was used alone or in combination. NPS that were use alone were considered. Each of the classes contained drugs that are modelled on existing illegal materials and are now covered by the UK New Psychoactive Substances Bill in 2016. Suggested Reviewers: Title Page (with authors and addresses) An overview of Emerging and New Psychoactive Substances in the United Kingdom Shruti Beharry and Simon Gibbons1 Research Department of Pharmaceutical and Biological Chemistry UCL School of Pharmacy -

Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj
Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj Nehad J. Ahmed1*, Menshawy A. Menshawy2 1Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia, 2Department of Medicinal chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia Abstract Aim: This study aims to illustrate the prescribing trends of antihistamines in the outpatient setting in Al-Kharj. Materials and Methods: This is a retrospective study that included the evaluation of antihistamines in the outpatient setting in a public hospital in Al-Kharj. The data were collected from the pharmacy-based computer system. Results: The total number of prescriptions that included antihistamines was 799. Most of the prescribed ORIGINAL ARTICLE ARTICLE ORIGINAL antihistamines were first-generation sedating antihistamines (chlorphenamine and diphenhydramine) (66.33%). About 63.20% of the prescribed antihistamines included chlorpheniramine followed by cetirizine (19.27%) and loratadine (14.39%). Conclusion: Antihistamines were prescribed commonly in the outpatient setting mainly first-generation sedating antihistamines. It is recommended to increase the awareness of health- care providers about the efficacy and the side effects of antihistamines and to encourage them to use these agents wisely. Key words: Antihistamines, outpatient, prescribing, trends INTRODUCTION In addition, antihistamines have been classified as sedating antihistamines (first-generation antihistamines) and non- ntihistamines are used in the sedating antihistamines (second-generation antihistamines).[4] management of allergic conditions. Sedating antihistamines include chlorphenamine, clemastine, They are useful for treating the itching hydroxyzine, alimemazine, cyproheptadine, promethazine, A [1] and ketotifen.[4] Non-sedating antihistamines include that results from the release of histamine. -

Octopamine and Tyramine Regulate the Activity of Reproductive Visceral Muscles in the Adult Female Blood-Feeding Bug, Rhodnius Prolixus Sam Hana* and Angela B
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 1830-1836 doi:10.1242/jeb.156307 RESEARCH ARTICLE Octopamine and tyramine regulate the activity of reproductive visceral muscles in the adult female blood-feeding bug, Rhodnius prolixus Sam Hana* and Angela B. Lange ABSTRACT Monastirioti et al., 1996). Octopamine and tyramine signal via The role of octopamine and tyramine in regulating spontaneous G-protein coupled receptors (GPCRs), leading to changes in second contractions of reproductive tissues was examined in the messenger levels. The recently updated receptor classification α β female Rhodnius prolixus. Octopamine decreased the amplitude of (Farooqui, 2012) divides the receptors into Oct -R, Oct -Rs β β β spontaneous contractions of the oviducts and reduced RhoprFIRFa- (Oct 1-R, Oct 2-R, Oct 3-R), TYR1-R and TYR2-R. In general, β α induced contractions in a dose-dependent manner, whereas tyramine Oct -Rs lead to elevation of cAMP while Oct -R and TYR-Rs lead to 2+ only reduced the RhoprFIRFa-induced contractions. Both octopamine an increase in Ca (Farooqui, 2012). and tyramine decreased the frequency of spontaneous bursal The movement of eggs in the reproductive system of contractions and completely abolished the contractions at Rhodnius prolixus starts at the ovaries, the site of egg maturation. 5×10−7 mol l−1 and above. Phentolamine, an octopamine receptor Upon ovulation, mature eggs are released into the oviducts antagonist, attenuated the inhibition induced by octopamine on the (Wigglesworth, 1942). Eggs are then guided, via oviductal oviducts and the bursa. Octopamine also increased the levels of peristaltic and phasic contractions, to the common oviduct, where cAMP in the oviducts, and this effect was blocked by phentolamine. -

Can Hepatic Coma Be Caused by a Reduction of Brain Noradrenaline Or Dopamine?
Gut: first published as 10.1136/gut.18.9.688 on 1 September 1977. Downloaded from Gut, 1977, 18, 688-691 Can hepatic coma be caused by a reduction of brain noradrenaline or dopamine? L. ZIEVE AND R. L. OLSEN From the Department ofMedicine, Minneapolis Veterans Hospital, University of Minnesota, Minneapolis, and Department of Chemistry, Hamline University, St. Paul, Minnesota, USA SUMMARY Intraventricular infusions of octopamine which raised brain octopamine concentrations more than 20 000-fold resulted in reductions in brain noradrenaline and dopamine by as much as 90% without affecting the alertness or activity of normal rats. As this reduction of brain catechol- amines is much greater than any reported in hepatic coma, we do not believe that values observed in experimental hepatic failure have aetiological significance for the encephalopathy that ensues. Though catecholaminergic nerve terminals represent dopamine and noradrenaline had no discernible only a small proportion of brain synapses (Snyder et effect on the state of alertness of the animals. al., 1973), the reduction in brain dopamine or nor- adrenaline by the accumulation of false neuro- Methods transmitterssuch as octopamine or of aromaticamino acids such as phenylalanine or tyrosine has been ANIMALS suggested as a cause of hepatic coma (Fischer and Male Sprague-Dawley rats weighing between 300 and Baldessarini, 1971; Dodsworth et al., 1974; Fischer 350 were g prepared by the method of Peterson and http://gut.bmj.com/ and Baldessarini, 1975; Munro et al., 1975). The Sparber (1974) for intraventricular infusions of following data have been cited in support of this octopamine. Rats of 250-400 g were used as controls. -

Structure-Cytotoxicity Relationship Profile of 13 Synthetic Cathinones In
Neurotoxicology 75 (2019) 158–173 Contents lists available at ScienceDirect Neurotoxicology journal homepage: www.elsevier.com/locate/neuro Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells T ⁎ Jorge Soaresa, , Vera Marisa Costaa, Helena Gasparb,c, Susana Santosd,e, Maria de Lourdes Bastosa, ⁎ Félix Carvalhoa, João Paulo Capelaa,f, a UCIBIO, REQUIMTE (Rede de Química e Tecnologia), Laboratório de Toxicologia, Departamento de Ciências Biológicas, Faculdade de Farmácia, Universidade do Porto, Portugal b BioISI – Instituto de Biossistemas e Ciências Integrativas, Faculdade de Ciências, Universidade de Lisboa, Portugal c MARE - Centro de Ciências do Mar e do Ambiente, Escola Superior de Turismo e Tecnologia do Mar, Instituto Politécnico de Leiria, Portugal d Centro de Química e Bioquímica (CQB), Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Portugal e Centro de Química Estrutural, Faculdade de Ciências, Universidade de Lisboa, Portugal f FP-ENAS (Unidade de Investigação UFP em Energia, Ambiente e Saúde), CEBIMED (Centro de Estudos em Biomedicina), Faculdade de Ciências da Saúde, Universidade Fernando Pessoa, Portugal ARTICLE INFO ABSTRACT Keywords: Synthetic cathinones also known as β-keto amphetamines are a new group of recreational designer drugs. We Synthetic cathinones aimed to evaluate the cytotoxic potential of thirteen cathinones lacking the methylenedioxy ring and establish a Classical amphetamines putative structure-toxicity profile using differentiated SH-SY5Y cells, as well as to compare their toxicity to that Cytotoxicity of amphetamine (AMPH) and methamphetamine (METH). Cytotoxicity assays [mitochondrial 3-(4,5-dimethyl-2- SH-SY5Y cells thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction and lysosomal neutral red (NR) uptake] per- Structure-toxicity relationship formed after a 24-h or a 48-h exposure revealed for all tested drugs a concentration-dependent toxicity. -

Your Child's Emergency Allergy Pack with Antihistamine
Your Child’s Emergency Allergy Pack with Antihistamine Patient information Paediatric Department Watford General Hospital Hemel Hempstead Hospital If you need this leaflet in another language, large print, Braille or audio version, please call 01923 217 187 or email [email protected] Author Dr Ashley Reece Department Paediatrics Ratified Date / Review Date Feb 2021 / Feb 2024 Version Number / ID Number 40-1104-V1 Why does my child I need an Allergy Action Pack? An allergy action pan is a kit contianing evrything you need in case of an allergic reaction. Is my/ my child’s allergy severe? There is no such thing as a mild or severe allergy as reactions are always unpredicatble. However we do classify reactions as mild, moderate or severe. While it follows that a severe reaction makes the anxiety about the severity of the next reaction high, we cannot predict the severity of subsequent reactions. However there are some foods and some allergies which are assessed as having very low risk of a significant reaction. The way to manage your child with their allergy is to ensure you have the best possible safety net in case of an unforeseen reaction. Your doctor will give you a specific allergy plan (like the one to the right) which you should also give to your child’s school. Generally allergy is mananged by: You MUST avoid any foods which you know your child is allergic to. Avoidance Take care with labels and risk assess any new foods and when eating out in a restaurant or from a take-away. -
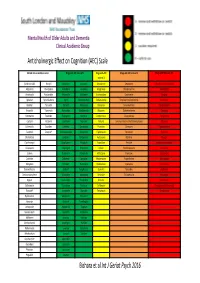
AEC Traffic Light System
Mental Health of Older Adults and Dementia Clinical Academic Group Anticholinergic Effect on Cognition (AEC) Scale Limited data so unable to score Drugs with AEC score of 0 Drugs with AEC Drugs with AEC score of 2 Drugs with AEC score of 3 score of 1 Alendronic Acid Ramipril Alprazolam Lovastatin Amiodarone Amantadine Alimemazine (trimeprazine) Allopurinol Rivaroxaban Amlodipine Lurasidone Aripiprazole Chlorphenamine Amitriptyline Anastrozole Rosuvastatin Amoxycillin Meloxicam Bromocriptine Desipramine Atropine Apixaban Spironolactone Aspirin Metoclopramide Carbamazepine Dicycloverine (dicyclomine) Benztropine Baclofen Tamoxifen Atenolol Metoprolol Citalopram Dimenhydrinate Chlorpromazine Bisoprolol Topiramate Atorvastatin Moclobemide Diazepam Diphenhydramine Clemastine Bumetanide Tizanidine Buproprion Morphine Domperidone Disopyramide Clomipramine Captopril Verapamil Cepahlexin Naproxen Fentanyl Levomepromazine (methotrimeprazine) Clozapine Carbimazole Zopiclone Cetirizine Omeprazole Fluoxetine Olanzapine Cyproheptadine Carvedilol Zotepine* Chlordiazepoxide Paracetamol Fluphenazine Paroxetine Dothiepin Chlortalidone Cimetidine Pantoprazole Hydroxyzine Pethidine Doxepin Clarithromycin Ciprofloxacin Pravastatin Iloperidone Pimozide Hyoscine hydrobromide Clonazepam Clopidogrel Propranolol Lithium Prochlorperazine Imipramine Codeine Darifenacin Rabeprazole Mirtazapine Promazine Lofepramine Colchicine Diclofenac Ranitidine Perphenazine Propantheline Nortriptyline Dabigatran Diltiazem Risperidone Prednisolone Quetiapine Orphenadrine Dexamethasone -

An Avoidable Cause of Thymoglobulin Anaphylaxis S
Brabant et al. Allergy Asthma Clin Immunol (2017) 13:13 Allergy, Asthma & Clinical Immunology DOI 10.1186/s13223-017-0186-9 CASE REPORT Open Access An avoidable cause of thymoglobulin anaphylaxis S. Brabant1*, A. Facon2, F. Provôt3, M. Labalette1, B. Wallaert4 and C. Chenivesse4 Abstract Background: Thymoglobulin® (anti-thymocyte globulin [rabbit]) is a purified pasteurised, gamma immune globulin obtained by immunisation of rabbits with human thymocytes. Anaphylactic allergic reactions to a first injection of thymoglobulin are rare. Case presentation: We report a case of serious anaphylactic reaction occurring after a first intraoperative injection of thymoglobulin during renal transplantation in a patient with undiagnosed respiratory allergy to rabbit allergens. Conclusions: This case report reinforces the importance of identifying rabbit allergy by a simple combination of clini- cal interview followed by confirmatory skin testing or blood tests of all patients prior to injection of thymoglobulin, which is formally contraindicated in patients with a history of hypersensitivity to rabbit proteins. Keywords: Thymoglobulin, Anaphylactic allergic reaction, Rabbit proteins Background [7]. Cases of serious anaphylactic reactions to thymo- Thymoglobulin is an IgG fraction purified from the globulin due to rabbit protein allergy are very rare, and serum of rabbits immunised against human thymocytes. consequently, specific tests for rabbit allergy are not The preparation consists of polyclonal antilymphocyte usually performed as part of the pre-transplant assess- IgG directed against T lymphocyte surface antigens, and ment. We report a case of serious anaphylactic reaction induction of profound lymphocyte depletion is though due to rabbit protein allergy following a first injection of to be the main mechanism of thymoglobulin-mediated thymoglobulin. -

Hypersensitivity Reactions (Types I, II, III, IV)
Hypersensitivity Reactions (Types I, II, III, IV) April 15, 2009 Inflammatory response - local, eliminates antigen without extensively damaging the host’s tissue. Hypersensitivity - immune & inflammatory responses that are harmful to the host (von Pirquet, 1906) - Type I Produce effector molecules Capable of ingesting foreign Particles Association with parasite infection Modified from Abbas, Lichtman & Pillai, Table 19-1 Type I hypersensitivity response IgE VH V L Cε1 CL Binds to mast cell Normal serum level = 0.0003 mg/ml Binds Fc region of IgE Link Intracellular signal trans. Initiation of degranulation Larche et al. Nat. Rev. Immunol 6:761-771, 2006 Abbas, Lichtman & Pillai,19-8 Factors in the development of allergic diseases • Geographical distribution • Environmental factors - climate, air pollution, socioeconomic status • Genetic risk factors • “Hygiene hypothesis” – Older siblings, day care – Exposure to certain foods, farm animals – Exposure to antibiotics during infancy • Cytokine milieu Adapted from Bach, JF. N Engl J Med 347:911, 2002. Upham & Holt. Curr Opin Allergy Clin Immunol 5:167, 2005 Also: Papadopoulos and Kalobatsou. Curr Op Allergy Clin Immunol 7:91-95, 2007 IgE-mediated diseases in humans • Systemic (anaphylactic shock) •Asthma – Classification by immunopathological phenotype can be used to determine management strategies • Hay fever (allergic rhinitis) • Allergic conjunctivitis • Skin reactions • Food allergies Diseases in Humans (I) • Systemic anaphylaxis - potentially fatal - due to food ingestion (eggs, shellfish,